
Our extensive preclinical in vivo research services are focused on delivering high quality drug discovery and drug development data to provide our clients with confidence when moving a new drug candidate forward to IND enabling GLP toxicology studies and to reach clinical trials. At Hera we are utilizing our gene editing technology to develop new preclinical animal models to decrease timelines and provide increased translatability and make an impact on the treatment of human diseases.
The SRG Rat model, the OncoRat®, is our unique fully SCID rat created using our gene editing technology, aimed at setting an entirely new standard for translational research, delivering higher xenograft tumor take-rate and more uniform tumor growth kinetics.
We offer a range of in vivo services to support your preclinical research needs, including xenograft and syngeneic tumor models, genetically engineered rat and mouse models, and pharmacology (PK/PD) studies. We also offer in vitro services, such as cell line gene editing and drug screening, to complete our comprehensive offerings. Our experienced team of scientists can work with you to design and execute studies that meet your specific research needs, whether in vivo, in vitro, or a combination of both.
Xenograft Model Services
We provide in vitro and in vivo oncology studies for drug discovery researchers in industry and academia. Our xenograft and PDX drug efficacy models are optimized in both traditional mouse strains and the SRG rat.
Our comprehensive range of xenograft and syngeneic tumor models for in vivo efficacy allow for the evaluation of potential any type of therapeutic. Our experienced team of scientists can work with you to choose the most appropriate model for your research needs.
Follow the link below to learn more about our in vivo oncology service offerings.
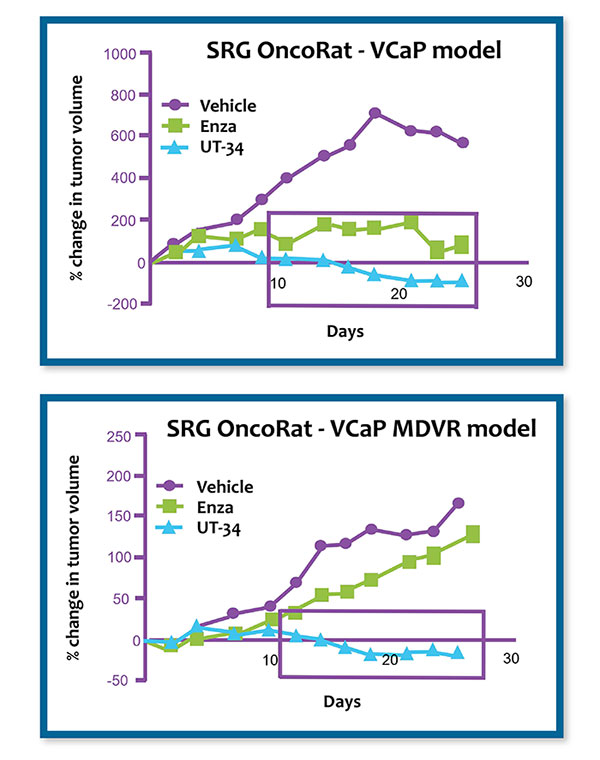

SRG OncoRat: Building a Better Trap for Cancer
SRG OncoRat, developed using Hera Biolabs’ advanced gene editing technology, is a SCID rat on the Sprague-Dawley background that harbors a double knockout for the Rag2 and Il2rgamma genes. Similar to industry leading NSG mice, OncoRat demonstrates enhanced immunodeficiency, lacking B-cells, T-cells, and NK-cells.
Combining these genetic changes in this immunodeficient rat allows the use of fewer animals through enhanced engraftment rates and improved tumor growth profiles for both cell-line tumor models and patient-derived xenografts (PDXs). OncoRat is Reliable, Efficient & Robust for combining efficacy, pharmacokinetic (PK), biomarker, and toxicology-related endpoints.
Immuno-Oncology Services
Partnering with us not only gives you access to unique SRG rat models but broadens your options with an experienced provider of mouse in vivo services on many backgrounds including NSG, NCG, NOG, and the nude mouse.
Our goal is to deliver the best data possible for our clients. We routinely use both rat and mouse for in vivo oncology and provide advanced immuno-oncology services in mice. Our immune-oncology platform uses mice engrafted with CD34+ hematopoietic stem/progenitor cells (HSPCs), a validated platform providing bone marrow engraftment and development of multi-lineage human immune cells.
We offer standardized humanized mouse models or your specific donor, whether HLA matching or engineered cells for your preclinical studies.
Advancing Personalized Medicine With SRG Rat Models
Goutham Narla, MD., PhD., and his team at the University of Michigan study the effects of modulating tumor suppressor gene activity. Specifically, he and his team of researchers are looking for small molecule activators of these genes to treat a wide range of cancers.
Recently published in PLOS One, Hera and Dr. Narla collaborated by using the OncoRat® to provide more personalized cancer treatments for patients. Through patient derived xenografts (PDX) using the OncoRat®, researchers can now grow and sequence PDX and patient tumors in tandem to see which arising mutations may sensitize the tumor to existing cancer drugs.
Watch the video below to learn more.
Related Resources
Find related and up-to-date information and literature for our In Vivo Oncology service
including blogs, posters, presentations, webinars, and white papers.
-
 Validation Of The SRG Rat For Human Tumor Studies Published In PLOS ONE
-
We Are Excited To Announce That A Validation Study Using […] Read More →
Validation Of The SRG Rat For Human Tumor Studies Published In PLOS ONE
-
We Are Excited To Announce That A Validation Study Using […] Read More →
-
 The OncoRat® Is The Ideal Host For Patient-Derived Xenografts Of Ovarian Cancer Cells
-
Ovarian cancer is the most lethal gynecological cancer in the […] Read More →
The OncoRat® Is The Ideal Host For Patient-Derived Xenografts Of Ovarian Cancer Cells
-
Ovarian cancer is the most lethal gynecological cancer in the […] Read More →
In Vivo vs In Vitro Models
In preclinical drug development, in vivo and in vitro studies are two important approaches used to evaluate the safety and efficacy of potential therapeutic agents. In vivo studies are conducted in living organisms, such as animals or humans, while in vitro studies are conducted outside of living organisms, typically using isolated cells or tissues.
In vivo (from the Latin for “within the living”) studies offer several advantages over in vitro studies, including the ability to evaluate the safety and efficacy of a therapeutic agent within a complex biological system. By studying the effects of a drug in in vivo experiments, researchers can gain a better understanding of how the drug interacts with different tissues and organs, as well as the potential side effects and toxicities associated with the drug. In vivo studies also allow for the evaluation of the drug’s pharmacokinetics and pharmacodynamics, which are critical factors in determining the appropriate dosing and administration of a drug.
In vitro testing, on the other hand, offer several advantages over in vivo studies, including greater control over experimental conditions and the ability to conduct high-throughput screening of potential therapeutic agents. In vitro studies also allow researchers to study the effects of a drug on specific cells, as cell culture, or tissues in isolation, which can provide valuable insights into the molecular mechanisms underlying the drug’s effects. Choosing the right preclinical animal model can be challenging making the jump from a petri dish or a test tube to live animal studies.
At Hera BioLabs, we offer a range of in vivo services to support your preclinical research needs, including xenograft and syngeneic tumor models, genetically engineered rat and mouse models, and pharmacology (PK/PD) studies. We also offer in vitro services, such as cell line gene editing and drug screening, to complete our comprehensive offerings. Our experienced team of scientists can work with you to design and execute studies that meet your specific research needs, whether in vivo, in vitro, or a combination of both.

