Chemically Induced Diabetes
Chemically induced obesity models provide reliable platform to study the underlying mechanisms of diabetes and related metabolic disorders and are an effective tool for testing novel therapeutics and interventions aimed at treating diabetes.
- Streptozotocin (STZ) induced type 1 diabetes.
- High fat diet (HFD) and STZ-induced type 2 diabetes Range finding or maximum tolerated dose studies
Cell Based Therapies in the SRG Rat
SRG OncoRat, developed using Hera Biolabs’ advanced gene editing technology, is a SCID rat on the Sprague-Dawley background that harbors a double knockout for the Rag2 and Il2rgamma genes. Similar to industry leading NSG mice, OncoRat demonstrates enhanced immunodeficiency, lacking B-cells, T-cells, and NK-cells.
Because the SRG rat is highly immunodeficient it is ideal for testing cell therapies in combination with standard methods of inducing an obesity or diabetes phenotype.
Available Assessments & Assays
Glucose Homeostasis
Insulin tolerance test (ITT; IP or IV)
Glucose tolerance test (GTT; PO, IP, or IV)
Glucose-stimulated insulin secretion (GSIS; PO, IP, or IV)
Food intake, water intake, fecal output, fecal bomb calorimetry
Body composition (carcass):
Whole-body chemical carcass analysis
Dual-energy X-ray absorptiometry (DXA)
Quantitative magnetic resonance (QMR)
Dyslipidemia:
Total cholesterol, HDL, & LDL
Triglycerides
Free fatty acids
ELISA, Luminex®, and Meso Scale Discovery for Hormone Quantification
RNA, Protein, and Lipid Isolation
Choosing the Right Preclinical Rodent Model for Obesity Research
Diabetes is a complex metabolic syndrome that affects millions of people worldwide. In preclinical research, selecting the appropriate animal model is crucial to studying the pathophysiology of diabetes and developing effective therapeutic interventions. Three commonly employed models include STZ-induced diabetes, diet-induced models, and genetically modified strains. Each model offers unique advantages and limitations, and understanding when to use them is essential for obtaining meaningful results.
STZ-Induced Diabetes Model: The streptozotocin (STZ)-induced diabetes model involves the administration of STZ, a naturally occurring compound that selectively destroys pancreatic beta cells, leading to insulin deficiency. This model mimics type 1 diabetes characterized by absolute insulin deficiency. STZ-induced diabetes is often used when studying autoimmune mechanisms, pancreatic beta cell transplantation, or evaluating novel therapeutic approaches aiming to restore insulin production. Researchers can control the severity of diabetes by adjusting the STZ dose, allowing for different degrees of beta cell destruction.
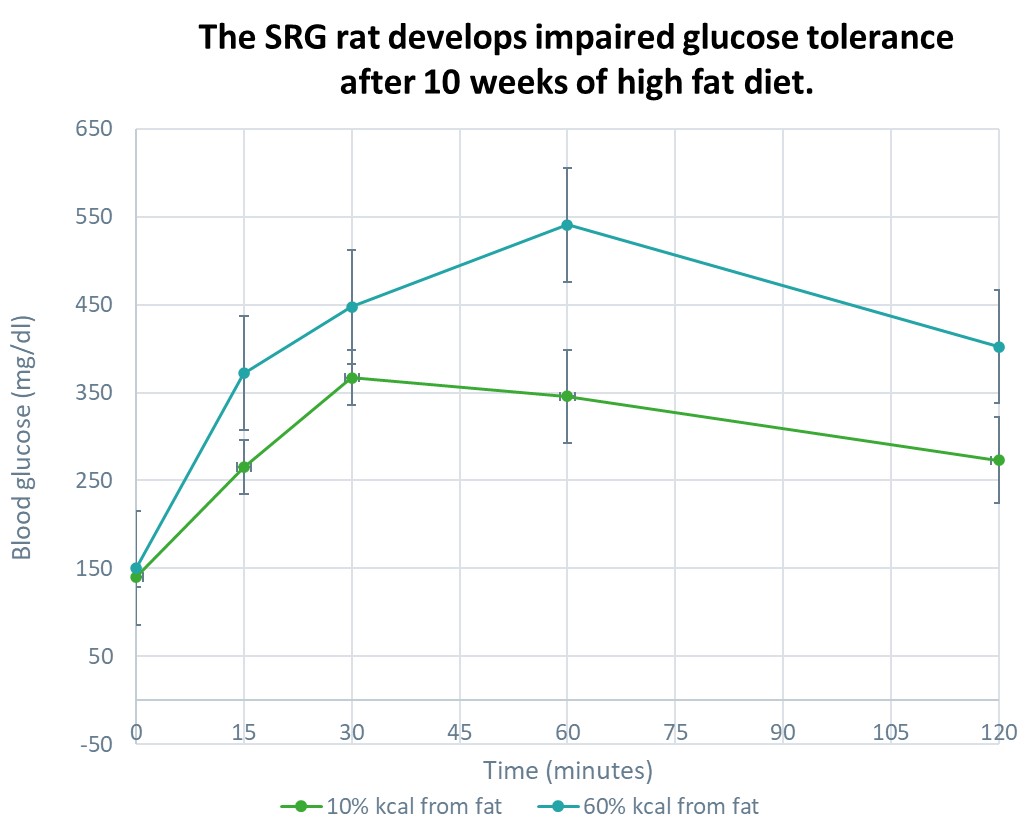
Diet-Induced Models: Diet-induced models involve feeding animals a high-fat or high-sugar diet, leading to obesity, insulin resistance, and eventual beta cell dysfunction. These models closely resemble type 2 diabetes, which is characterized by insulin resistance and relative insulin deficiency. Diet-induced models are versatile and widely used, as they reflect the lifestyle and dietary factors contributing to the development of type 2 diabetes in humans. They are particularly useful for studying metabolic abnormalities, glucose homeostasis, and exploring the effects of dietary interventions or pharmacological treatments.
Genetically Modified Strains: Genetically modified strains involve altering the genetic makeup of animals to mimic specific aspects of diabetes, such as impaired insulin signaling, defective beta cell function, or disrupted glucose metabolism. These models enable researchers to investigate the precise molecular mechanisms underlying diabetes. Genetically modified strains are valuable for understanding the genetic basis of diabetes and evaluating potential therapeutic targets. However, it is important to note that these models may not fully capture the complexity of the human disease and should be complemented with other models for comprehensive insights.
The choice of model depends on the specific research question and the aspect of diabetes being investigated and considering the research objectives, disease phenotype, and translational relevance, researchers can select the most appropriate model or you can let our scientific team provide expertise on the best model for your research goals.
Additional considerations:


