Meet Your Research Partner
What Drives Our Work
Hera provides your research
program with:
Our Story
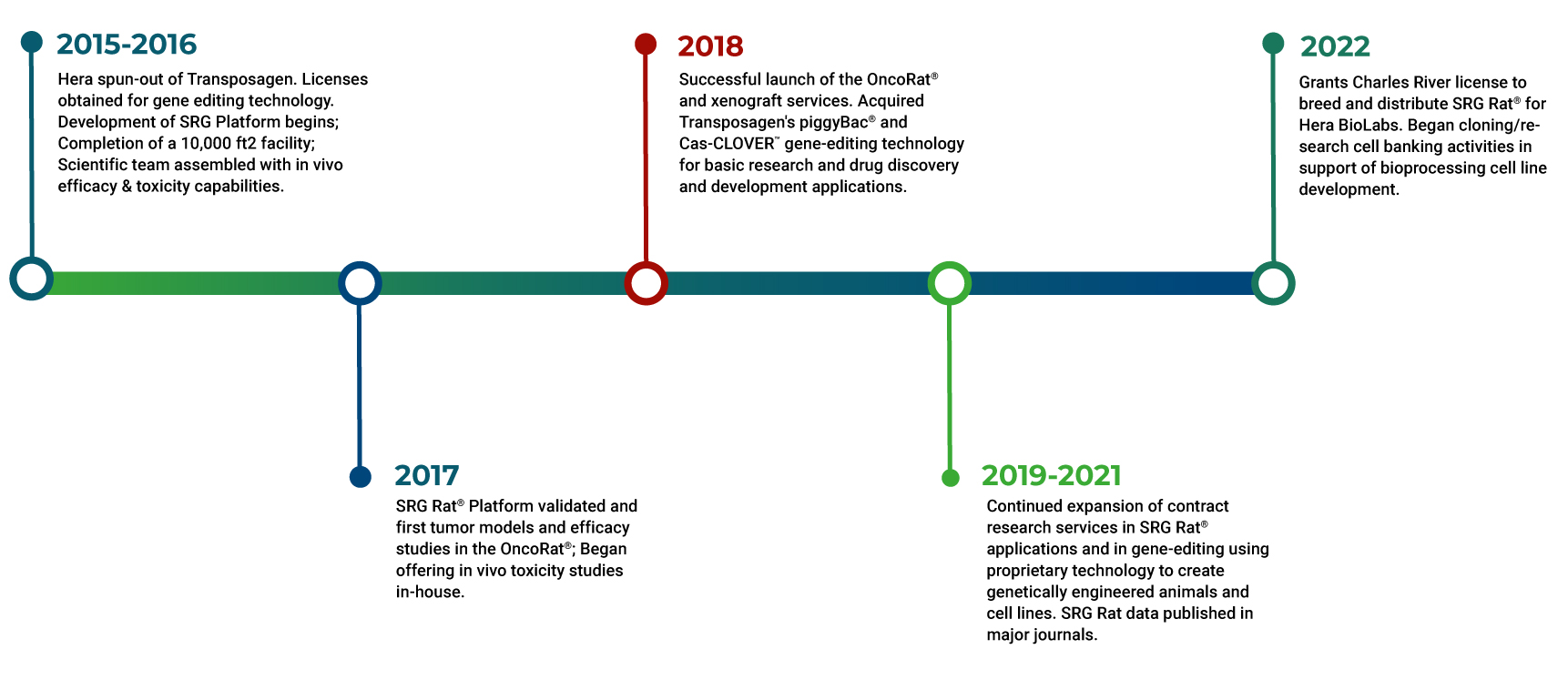
Hera Biolabs is an innovative preclinical contract service, products, and licensing provider to the life sciences industry. Born from cutting-edge gene editing technology developed by Transposagen Biopharmaceuticals, Hera forged its own path in 2015 to bring novel models and cell-line engineering expertise to early-stage drug development researchers.
In the following years, we used our proprietary technologies to build the SRG Rat® platform, a double knockout Sprague-Dawley rat engineered for T-cell, B-cell, and NK cell deficiency. In 2017 we introduced our first SRG Rat® model, the SRG OncoRat®, to researchers as a preferable alternative to traditional oncology studies in mice. The OncoRat® was quickly adopted by major research institutions and industry thanks to its larger size, excellent tumor take-rates, and smooth transition from efficacy to safety studies. To bring this improved model to the global research community, we partner with Charles River for breeding and distribution but remain the experts and only fully licensed contract research organization for SRG Rat® model users.
Today, we are continuing to expand our pipeline of models and services to support critical indications from our state-of-the-art labs and AAALAC-accredited animal research facility in Lexington, Kentucky, USA.

Meet Our Experts
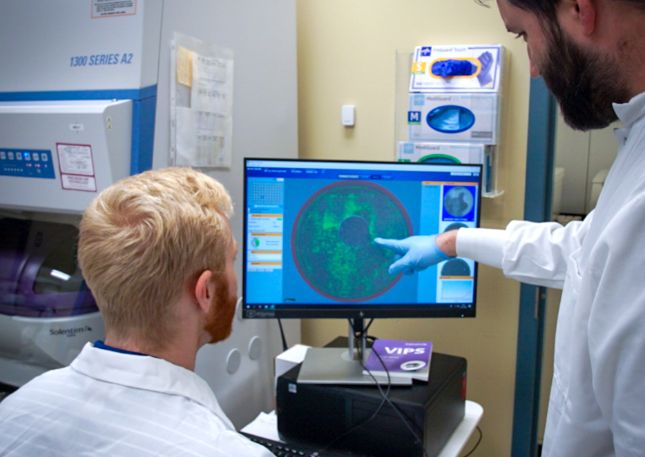
Our team brings decades of versatile expertise across gene editing, animal services, and related analytical testing. Combined with a passion for advancing human health and happiness, your drug discovery and development efforts are in good hands!
Current Innovations
Our mission to address the translatability gap from basic drug development to clinical trials through novel model creation does not stop with the OncoRat®. Every day, our experts combine the capabilities of our Cas-CLOVER™ Site-Specific Gene Editing System and Non-viral piggyBac® DNA Delivery System to expand our robust pipeline of animal models and associated services.
Our current rat model creation efforts focus on supporting immuno-oncology indications with a humanized SRG Model – the ImmunoRat™ (expected in 2025) and, in pharmacology and toxicology, through a humanized liver SRG Model – HepatoRat™ (expected in 2025).
Hera is leveraging our gene editing platforms and experience to address biomanufacturing difficulties in the early stages of protein expression by engineering host bioproduction CHO and HEK-293 cell line platforms to improve protein expression, scalability, and post-translation modifications. Our Cell Line Development (CLD) services are based on our optimized CHO platform which provides improved protein titers, long-term stability, and decreased timelines.