Targeting KRAS In Oncology Research?
KRAS has become an important drug target in multiple cancers due to its central role in hypertrophic and pro-mitotic signaling. Hera provides several KRAS mutant xenograft cell line models in the SRG rat for researchers developing drug candidates targeting KRAS. KRAS is a small GTPase that is activated in response to growth factors including HGF, TGFα, and EGF. KRAS then activates the MAP kinase (MAPK; RAF/MEK/ERK) signaling pathway, driving ERK translocation to the nucleus, and resulting in transcription of pro-mitotic and pro-growth genes. Additionally, KRAS contributes to PI3K/AKT/mTOR signaling, leading to transcriptional activation as well as hypertrophic protein synthesis via mTOR1 (figure 1).
Activating mutations in one of the three RAS genes (HRAS, NRAS, and KRAS) have been observed in up to 20% of all tumors, with mutations in the KRAS gene observed in ~90% of pancreatic cancers, ~50% of colorectal cancers, and ~25% non-small cell lung cancers (NSCLC)1. The G12D mutation is most common in pancreatic and the G12C mutation is most common in NSCLC, while many colon cancers harbor both the G12D and G13 KRAS mutations2.
What Experimental Models Most Closely Replicate Human Tumors?
Heated interest in KRAS as an anticancer target has led to the development of numerous KRAS inhibitors such as Amgen’s Lumakras (sotorasib), recently approved for the treatment of NSCLC3. Although KRAS was first described in 1983, efforts to develop clinically effective KRAS inhibitors were largely unsuccessful until recently. Production of KRAS inhibitors has been stymied by lack of consistency in methods, reagents, and protein expression systems, with many groups using truncated KRAS proteins that exhibit altered tertiary structure and lack cell membrane interactions4. Furthermore, sensitivity to KRAS inhibition is highly cell-type specific, and numerous studies indicate that inhibition at multiple points in the KRAS signaling cascade may be necessary to achieve clinically relevant effects1. Thus, patient-derived xenografts and stable cell lines expressing full length KRAS G12C, G12D, and G13 mutant proteins are required for preclinical evaluations of KRAS inhibition.
Hera BioLabs’ SRG OncoRat® is immunodeficient and highly permissive to human xenografts and cell line inoculation. Developed on a Sprague-Dawley background, SRG OncoRats are Rag2/Il2rg double knockouts that lack mature B cells, T cells, and circulating NK cells5. Compared to immune-deficient mouse models, the SRG OncoRat® provides the following advantages:
- Allows hosting for larger tumors (up to 10x volume compared to mice)
- Increased tumor take-rates
- Increased tumor growth kinetics
- Serial blood and tissue sampling for PK/PD studies
- Allows for the collection of efficacy data in the relevant toxicology/metabolism Sprague Dawley strain.
Which KRAS Mutant Cell Lines Have Been Successfully Grafted Into The SRG OncoRat®?
To date, Hera BioLabs has validated four KRAS mutant lines in the SRG OncoRat®: H358, HCT-116, MIA PaCa-2, and Capan-2 (table 1).
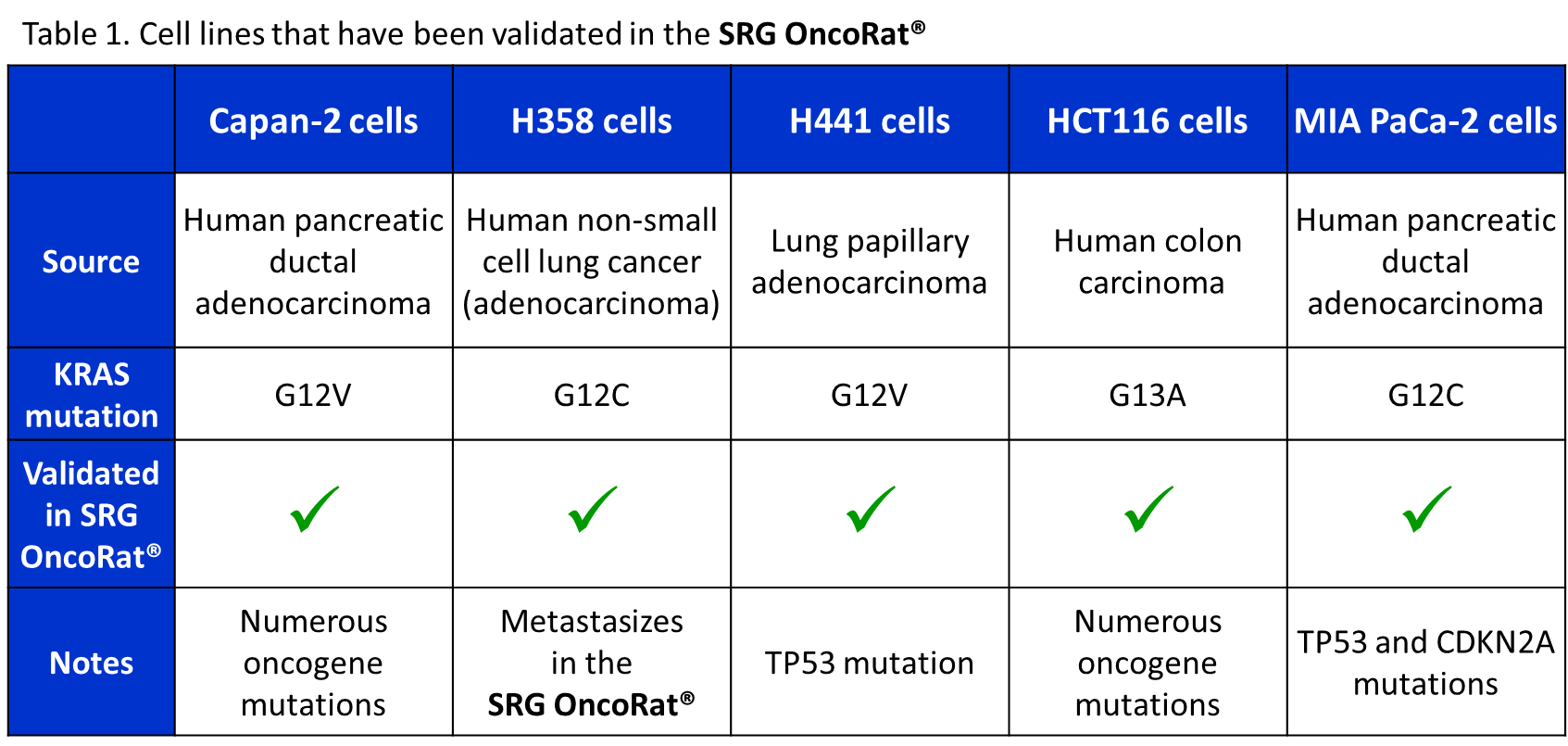
Capan-2, a human pancreatic ductal adenocarcinoma cell line, display epithelial morphology when grown in adherent tissue culture. Following xenograft into animals, Capan-2 form well-differentiated tumors. They express mutant KRAS (G12V) and elevated levels of the Epidermal Growth Factor Receptor (EGFR). In addition, they express wild-type p53 and normal levels of SMAD4 protein. When injected into immunocompromised SRG rats, they form well-differentiated tumors and are used as a PDX model for pancreatic cancer. The Capan-2 cells express mutant K-Ras (G12V), elevated Epidermal Growth Factor Receptor (EGFR), wild-type p53 and normal levels of the SMAD4 protein6. Figure 2 shows robust capan-2 tumor growth after inoculation into the SRG OncoRat®.
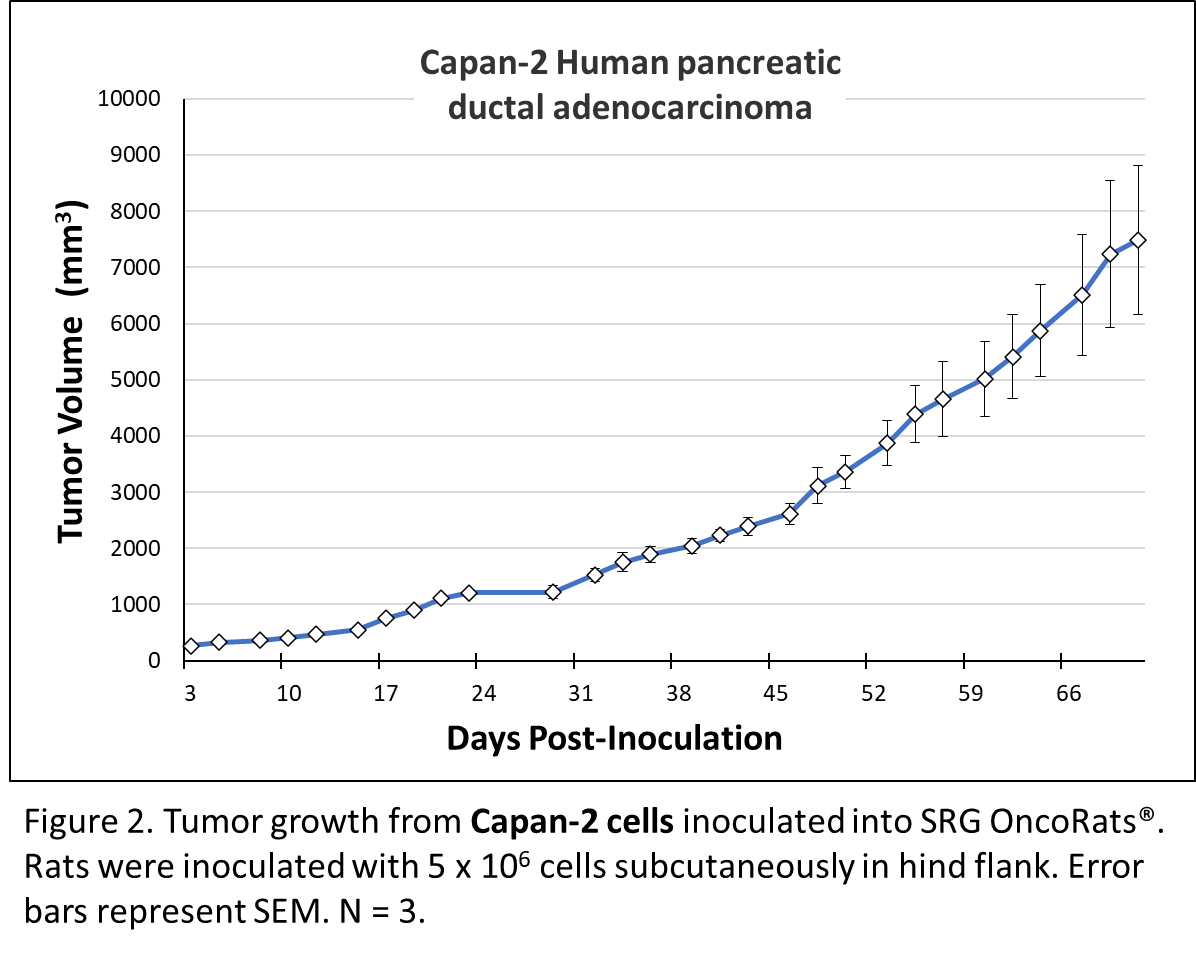
H358 NSCLC cells contain cytoplasmic structures that are characteristic of Club cells. These cells only have the KRAS G12C mutation, without any other tumor suppressor or oncogenic mutations and they are exceptionally useful for the study of KRAS, EGFR, BRAF, MEK, and ERK signaling. Despite their KRAS mutation, H358 cells are sensitive to anti-GFR therapies. These cells were derived from a metastatic site7, and our preliminary data indicate that H358 cells form metastases in the SRG OncoRat®. Tumor growth in the SRG OncoRat® is shown in Figure 3.
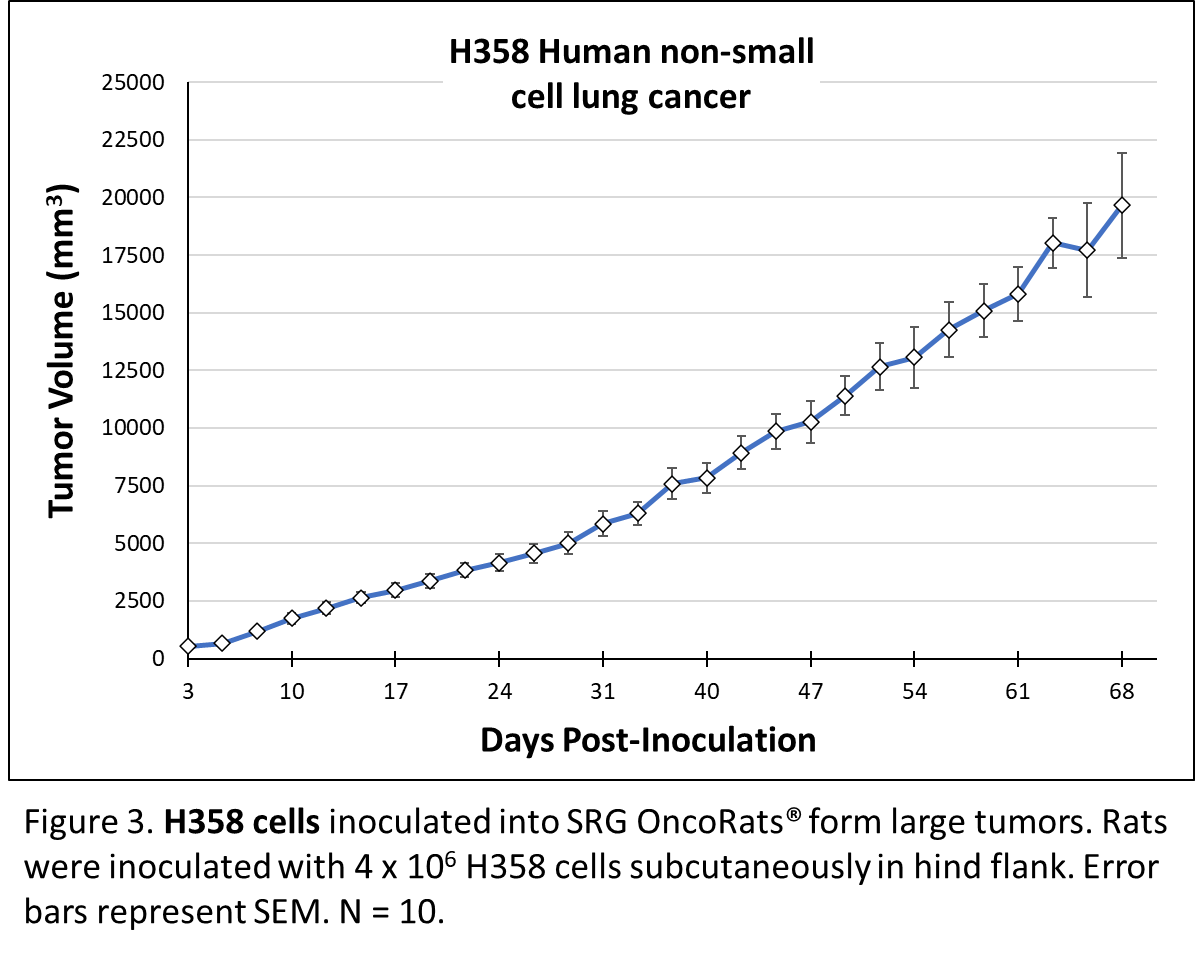
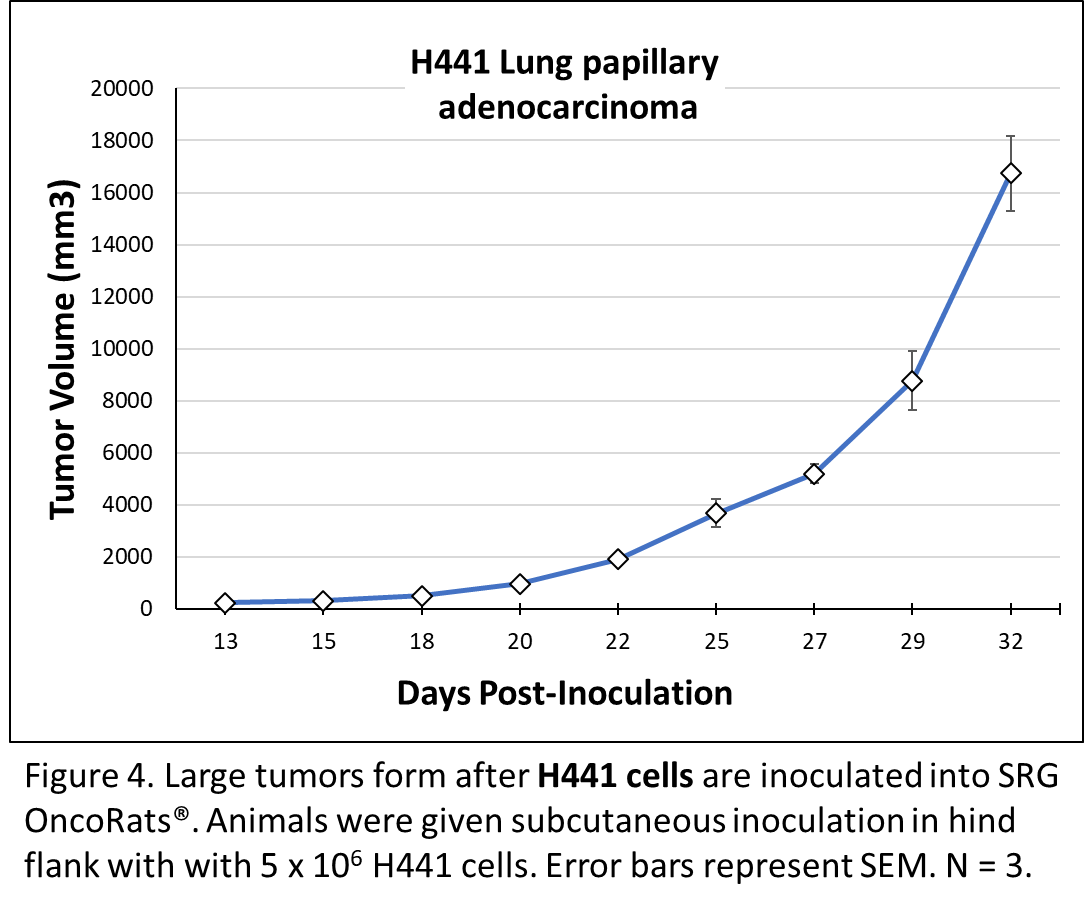
H441 Lung papillary adenocarcinoma cells were derived from a pericardial effusion metastasis in a 33-year-old man. In vitro, H441 form monolayers with epithelial barrier properties. These cells also harbor the TP53 A158L mutation and express surfactant protein A (SP-A). Like H358, H441 cells contain cytoplasmic structures that are characteristic of Club cells7,8. H441 inoculation induces robust tumor growth in the SRG OncoRat®, as shown in Figure 4.
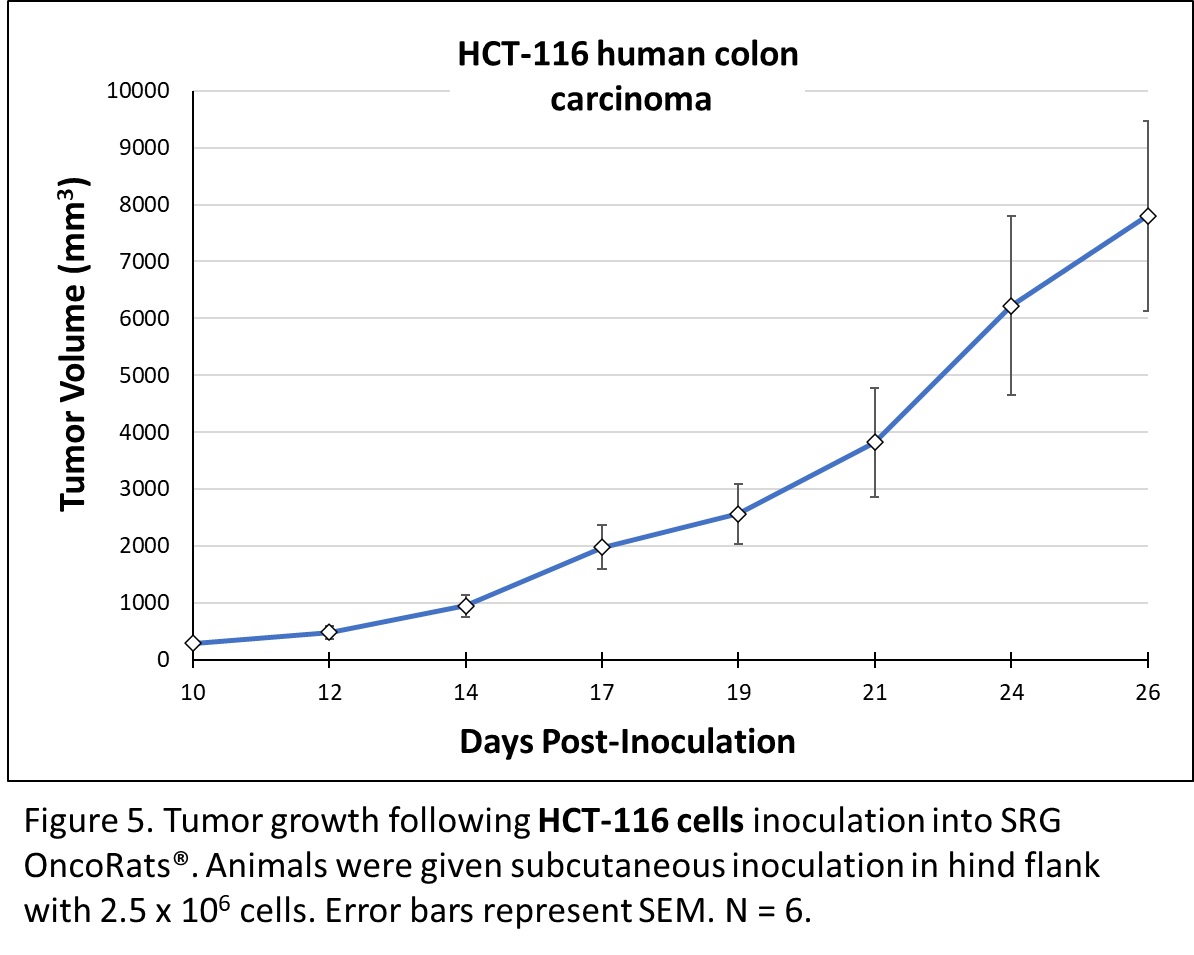
HCT-116 human colon cancer cells display epithelial morphology and have been extensively characterized in proliferation, tumorigenicity, and drug screening studies. In vitro, HCT-116 are highly motile and subcutaneous xenografts in nude mice have demonstrated them to be highly tumorigenic9. When grafted into the SRG OncoRat®, tumor take rate was 100% and tumor volumes reached 1800 to 12,000 mm3 by 24 days post-inoculation. When equal cell volumes were inoculated into NSG mice and SRG OncoRats® tumors were roughly 5-fold larger in the rats (figure 5)5.
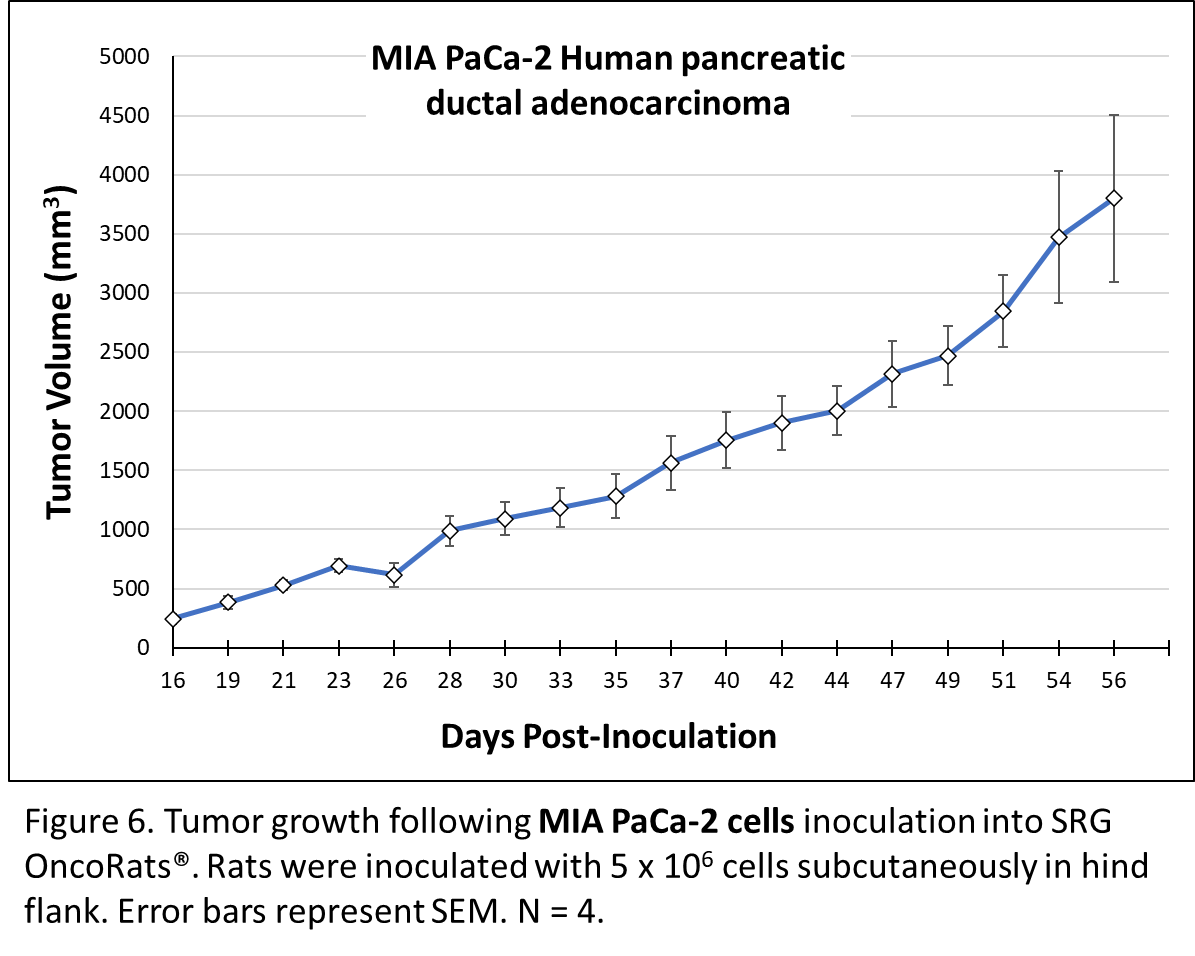
MIA PaCa-2 pancreatic ductal adenocarcinoma cells were obtained from a 65-year-old male. These cells are extremely well-characterized, with 1235 entries in PubMed. MIA PaCa-2 harbor TP53 mutations and homozygous deletions in the first 3 exons of CDKN2A/p16INK4A, without SMAD4/DPC4 mutations or microsatellite instability10. These cells express human colony stimulating factor, subclass I (CSF-I) and plasminogen activator. In vitro cells are adherent, with epithelial morphology. Subcutaneous inoculation with MIA PaCa-2 in the SRG OncoRat® yielded consistent tumor growth (figure 6)5.
Targeting KRAS Mutant Positive Cancers With The SRG OncoRat®
The SRG OncoRat® is a powerful tool for in vivo oncology studies, as it performs better than mouse models in several key ways. Our rats are extremely well-suited for hosting PDX and CDX cancer lines for analyses, including drug efficacy, tumor growth kinetics, and PK/PD studies. Tumor volumes are far larger than those obtained in mice, allowing for more detailed molecular characterization of tumors as well as PDX banking.
These qualities also make the SRG OncoRats® attractive candidates for use as patient avatars, wherein
personalized precision therapies can be rapidly tested in vivo to guide clinical decision making.
If your preclinical studies could use a boost, or you would like to see more data on the SRG Rat, contact Hera BioLabs for more information here.
References
- Molina-Arcas, M., Samani, A. & Downward, J. Drugging the Undruggable: Advances on RAS Targeting in Cancer. Genes (Basel) 12, doi:10.3390/genes12060899 (2021).
- Hobbs, G. A., Der, C. J. & Rossman, K. L. RAS isoforms and mutations in cancer at a glance. J Cell Sci 129, 1287-1292, doi:10.1242/jcs.182873 (2016).
- Dunnett-Kane, V., Nicola, P., Blackhall, F. & Lindsay, C. Mechanisms of Resistance to KRAS(G12C) Inhibitors. Cancers (Basel) 13, doi:10.3390/cancers13010151 (2021).
- Esposito, D., Stephen, A. G., Turbyville, T. J. & Holderfield, M. New weapons to penetrate the armor: Novel reagents and assays developed at the NCI RAS Initiative to enable discovery of RAS therapeutics. Semin Cancer Biol 54, 174-182, doi:10.1016/j.semcancer.2018.02.006 (2019).
- Noto, F. K. et al. The SRG rat, a Sprague-Dawley Rag2/Il2rg double-knockout validated for human tumor oncology studies. PLoS One 15, e0240169, doi:10.1371/journal.pone.0240169 (2020).
- Kyriazis, A. A., Kyriazis, A. P., Sternberg, C. N., Sloane, N. H. & Loveless, J. D. Morphological, biological, biochemical, and karyotypic characteristics of human pancreatic ductal adenocarcinoma Capan-2 in tissue culture and the nude mouse. Cancer Res 46, 5810-5815 (1986).
- Brower, M., Carney, D. N., Oie, H. K., Gazdar, A. F. & Minna, J. D. Growth of cell lines and clinical specimens of human non-small cell lung cancer in a serum-free defined medium. Cancer Res 46, 798-806 (1986).
- Salomon, J. J. et al. The cell line NCl-H441 is a useful in vitro model for transport studies of human distal lung epithelial barrier. Mol Pharm 11, 995-1006, doi:10.1021/mp4006535 (2014).
- Rajput, A. et al. Characterization of HCT116 human colon cancer cells in an orthotopic model. J Surg Res 147, 276-281, doi:10.1016/j.jss.2007.04.021 (2008).
- Gradiz, R., Silva, H. C., Carvalho, L., Botelho, M. F. & Mota-Pinto, A. MIA PaCa-2 and PANC-1 – pancreas ductal adenocarcinoma cell lines with neuroendocrine differentiation and somatostatin receptors. Sci Rep 6, 21648, doi:10.1038/srep21648 (2016).
