Around 85% of preclinical drugs entering clinical trials in oncology never make it past Phase 1 because they fail to demonstrate sufficient efficacy due to testing in unselected patient populations 1-3. Many of these failed agents are targeted therapies, meaning that they target specific oncogenic driver mutations. However, not all tumors harbor the same, or even similar, driving mutations – these targeted therapies are often found to be beneficial in very small patient populations which would not be captured in an unselected-patient trial design.
This high failure rate is primarily due to two limitations of preclinical targeted therapy research:
- The lack of preclinical biomarker identification leads to study design without patient selection. Sub-populations of patients where the drug is efficacious are not identified, and this often leads to the failure of the drug.
- Due to poor engraftment rates, immunodeficient mice lack the ability to establish patient derived xenografts that represent the heterogeneity of mutational burden within tumor-cell populations and the clinical behavior of human tumor. This limits the ability to identify clinically relevant biomarkers and tumor characteristics for accurate study design.
Both of these limitations can be overcome by using patient derived xenografts (PDX) from wide varieties of the target patient population to determine preclinical drug efficacy and identify biomarkers of drug response. Patient derived xenografts are generated by implanting resected human tumor tissue, taken as fresh or frozen biopsies, into immunodeficient mice or rats. Patient derived xenografts are then grown in the murine host until they can be divided and implanted into multiple animals allowing for the study of multiple variables on the same tumor sample.
Patient derived xenografts maintain much of the histological characteristics of the human tumor including the tumor microenvironment, degree of differentiation, and the heterogeneity of the tumor cell population 2, 4. This method best models the original host tumor and subsequent drug activity for preclinical study making them a fundamental tool for oncology research, drug development and precision medicine initiatives.
Importantly, patient derived xenografts have been shown to accurately predict patient response to targeted therapies and the ability to expand and subdivide patient derived xenografts into multiple animals allows for the testing of different targeted therapies or combinations of therapies on the tumor of the same patient3. When this is coupled with information about the genomic or molecular characteristics of tumors, biomarkers of drug-response can be identified concurrently or retrospectively with patient treatment. For these reasons, it has been suggested that patient derived xenografts could be an invaluable preclinical tool to guide clinical trial design, patient selection and influence therapeutic decisions 3.
While promising, fully realizing the value of patient derived xenografts for preclinical trial work has many shortcomings due to limitations of the murine model they were first developed in. Mice were originally used for the development of patient derived xenograft models due to the plethora of immunodeficient mouse strains available at the time. However, using mice as hosts for patient derived xenografts has been a limitation of the technique since its inception.
Some of the limitations of using mice for these studies include:
- Mice have very low patient derived xenograft engraftment rates, often 5-20% or less, making it difficult to generate patient derived xenograft models that represent the population5.
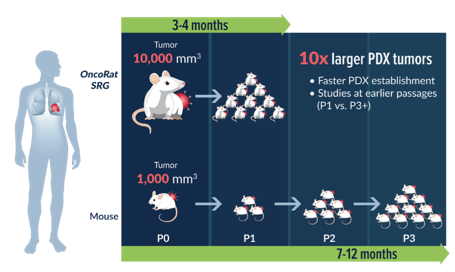
- Mice can support relatively small tumors limiting the amount of tissue and increasing the number of passages needed to generate enough tissue for study. This can make the study of a single tumor take 7-12 months or longer
- Growth kinetics of patient derived xenografts in mice vary greatly making it difficult to develop cohorts of animals with similar tumor volumes for efficacy studies.
- Multiple passages and long growth periods leads to loss of histological characteristics of the parent tumor, cell population selection and loss of heterogeneity amongst tumor cells when compared to the parent patient derived xenograft 6.
To overcome each of these limitations, Hera has generated the OncoRat® on the SRG™ platform. The OncoRat has been validated by Hera and collaborators for patient derived xenograft engraftment and studies.
Using rats as hosts for patient derived xenografts is superior to mice as they overcome the greatest limitations of the mouse model:
- The OncoRat boasts an engraftment take rate of 90%+ using non-small cell lung cancer (NSCLC) patient derived xenograft model establishment as the example.

- The OncoRat is able to generate tumors with volumes greater than ten times what can be generated in a mouse model allowing for efficacy studies to commence as early as passage1

- Growth kinetics of patient derived xenografts are more uniform across OncoRat animals when compared to mice.
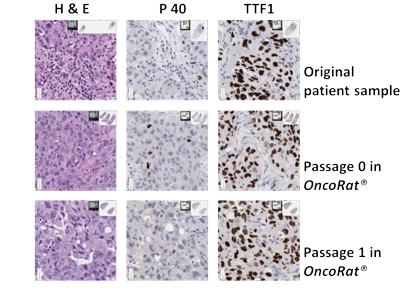
- Low number of passages and shorter growth periods needed to initiate efficacy studies using the OncoRat allowed the patient derived xenograft to maintain heterogeneity, genomic integrity and histological characteristics of the parent tumor sample
References
- Arrowsmith, J.; Miller, P., Trial watch: phase II and phase III attrition rates 2011-2012. Nature reviews. Drug discovery 2013, 12 (8), 569.
- DiMasi, J. A.; Reichert, J. M.; Feldman, L.; Malins, A., Clinical approval success rates for investigational cancer drugs. Clinical pharmacology and therapeutics 2013, 94 (3), 329-35.
- Gao, H.; Korn, J. M.; Ferretti, S.; Monahan, J. E.; Wang, Y.; Singh, M.; Zhang, C.; Schnell, C.; Yang, G.; Zhang, Y.; Balbin, O. A.; Barbe, S.; Cai, H.; Casey, F.; Chatterjee, S.; Chiang, D. Y.; Chuai, S.; Cogan, S. M.; Collins, S. D.; Dammassa, E.; Ebel, N.; Embry, M.; Green, J.; Kauffmann, A.; Kowal, C.; Leary, R. J.; Lehar, J.; Liang, Y.; Loo, A.; Lorenzana, E.; Robert McDonald Iii, E.; McLaughlin, M. E.; Merkin, J.; Meyer, R.; Naylor, T. L.; Patawaran, M.; Reddy, A.; Röelli, C.; Ruddy, D. A.; Salangsang, F.; Santacroce, F.; Singh, A. P.; Tang, Y.; Tinetto, W.; Tobler, S.; Velazquez, R.; Venkatesan, K.; Von Arx, F.; Wang, H. Q.; Wang, Z.; Wiesmann, M.; Wyss, D.; Xu, F.; Bitter, H.; Atadja, P.; Lees, E.; Hofmann, F.; Li, E.; Keen, N.; Cozens, R.; Jensen, M. R.; Pryer, N. K.; Williams, J. A.; Sellers, W. R., High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nature medicine 2015, 21, 1318.
- Cassidy, J. W.; Caldas, C.; Bruna, A., Maintaining Tumour Heterogeneity in Patient-Derived Tumour Xenografts. Cancer research 2015, 75 (15), 2963-2968.
- Wang, Y.; Lin, D.; Gout, P. W., Patient-Derived Xenograft Models of Human Cancer. Cham : Springer International Publishing : Imprint: Humana Press: 2017.
- Lee, J. Y.; Kim, S. Y.; Park, C.; Kim, N. K. D.; Jang, J.; Park, K.; Yi, J. H.; Hong, M.; Ahn, T.; Rath, O.; Schueler, J.; Kim, S. T.; Do, I.-G.; Lee, S.; Park, S. H.; Ji, Y. I.; Kim, D.; Park, J. O.; Park, Y. S.; Kang, W. K.; Kim, K.-M.; Park, W.-Y.; Lim, H. Y.; Lee, J., Patient-derived cell models as preclinical tools for genome-directed targeted therapy. Oncotarget 2015, 6 (28), 25619-25630.
