The American Cancer Society estimates that approximately 60,650 new leukemia cases and 24,000 leukemia deaths will occur in 2022. Of these, acute myeloid leukemia (AML) will account for around 20,050 new cases and 11,540 deaths. Almost all will be in adults, with increased risk associated with age and male sex1. AML, the most common leukemia in adults, involves uncontrolled proliferation of myeloid stem or progenitor cells in hematopoietic tissue. These aberrant myeloid cells accumulate in the bone marrow, leading to decreased hematopoiesis, thrombocytopenia and anemia2.
Left untreated, AML progresses rapidly to death. Thus, it is imperative that pharmacological interventions work rapidly. Due to this constraint, it is believed that AML patients have often received higher than needed drug doses. Recently, the US FDA released Project Optimus, a set of guidelines aimed at refining dose optimization in the clinical setting3. The guideline has already impacted clinical pipelines for AML therapy; Kura has announced a dose-optimization trial for their AML drug KO-539 and Amgen will soon do the to do the same with their KRAS inhibitor Lumakras4.
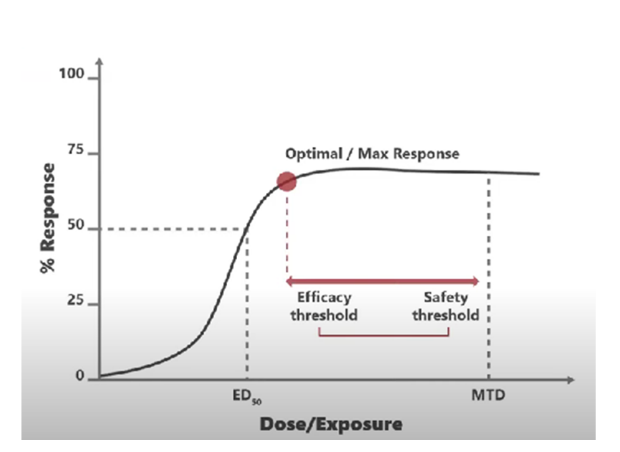
Figure 1. Bracketing Studies use more dose level groups in order to provide more information about the relationships between efficacy, safety, toxicity, and dose.
The FDA also recommends that drug developers consider Project Optimus when designing pre-clinical studies. The FDA would like to see more dose levels tested in animals, with an emphasis on assessing tumor drug exposure and performing bracketing analyses to determine optimal dosing (Figure 1).
The SRG OncoRat offers numerous advantages for performing pre-clinical AML studies in keeping with Project Optimus recommendations. Due to its larger size, the SRG rat allows for multiple types of data to be obtained from the same animals within efficacy studies, including:
- Serial blood draws for Pharmacokinetics & pharmacodynamics
- Serial tumor biopsies for tracking tumor drug exposure over time
- Better take rate with difficult tumor models, such as AML
- Lower variance in tumor growth kinetics, allowing for fewer animals and more doses
We have recently developed a solid tumor AML model in the SRG rat, allowing for maximum use of each animal. The MOLM-13 cell line was established from the blood of a young Japanese man with relapsing acute myeloid leukemia that progressed from myelodysplastic syndrome. These cells express monocyte-specific esterase, MLL-AF9 fusion mRNA, trisomy 8, as well as other trisomies that are present in various subclones5. MOLM-13 tumors exhibit rapid growth, and they are responsive to Sponsor test articles.
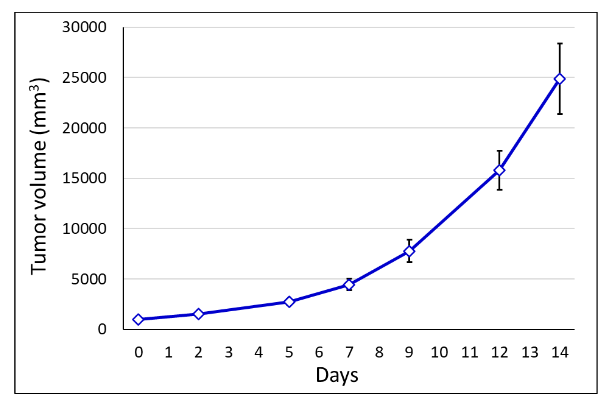
Figure 2. MOLM-13 tumor growth. SRG rats were inoculated subcutaneously with 75,000 MOLM-13 cells and tumor growth was monitored with electronic calipers. N = 9.
The SRG OncoRat is a powerful tool for in vivo oncology studies, as it outperforms mouse models for compliance with the goals of Project Optimus. SRG rats are extremely well-suited for hosting solid AML tumors for analyses, including drug efficacy, tumor growth kinetics, and PK/PD studies. Tumor volumes are far larger than those obtained in mice, allowing for serial tumor biopsies to assess drug exposure.
If your preclinical studies could use a boost, or you would like to see more data on the SRG Rat, contact Hera BioLabs for more information here.
References
- Cancer Facts & Figures 2022, https://www.cancer.org/cancer/acute-myeloid-leukemia/about/key-statistics.html#references (2022).
- Almosailleakh, M. & Schwaller, J. Murine Models of Acute Myeloid Leukaemia. Int J Mol Sci 20, doi:10.3390/ijms20020453 (2019).
- Project Optimus: Reforming the dose optimization and dose selection paradigm in oncology, https://www.fda.gov/about-fda/oncology-center-excellence/project-optimus (2022).
- Armstrong, A. FDA’s renewed focus on oncology dosing spooks investors, but companies say they’re ready, https://www.fiercebiotech.com/biotech/fda-s-renewed-focus-oncology-dosing-spooks-investors-but-companies-say-they-were-ready (2021).
- Matsuo, Y. et al. Two acute monocytic leukemia (AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia 11, 1469-1477, doi:10.1038/sj.leu.2400768 (1997).
