A case study of SRG OncoRat tumor xenografts from a Clinical Cancer Research publication
Bridging The ‘valley of death’ In Oncology Clinical Trials
While drug development pipelines have generated many valuable therapies, a significant number of clinical trials fail. Recent estimates put overall probability of success (POS) for clinical trials at ~13.8%, but this varies widely dependent on the therapeutic area.(1) In particular, over the course of 17,368 total cancer clinical trials from 2000 to 2015, the POS was ~3.4%, the lowest of all the therapeutic areas. This indicates particularly poor translatability of preclinical oncology data to humans. As the median time to completion for oncology trials are approximately twice that of non-oncology trials, the opportunity cost of pursuing a dead-end drug are even greater. Tools for improving preclinical relevance and bridging the so-called “valley of death” between basic research and novel therapeutics are desperately needed.(2)
Using the SRG OncoRat and xenograft validation services, researchers were able to:
- Collect and validate an efficient, human- relevant model system
- Determine lead compound efficacy towards human prostate tumors
- Publish research data in a high-impact clinical journal (Clinical Cancer Research) -Collect a valuable asset for potential IND
OncoRat: Building A Better Trap For Cancer.
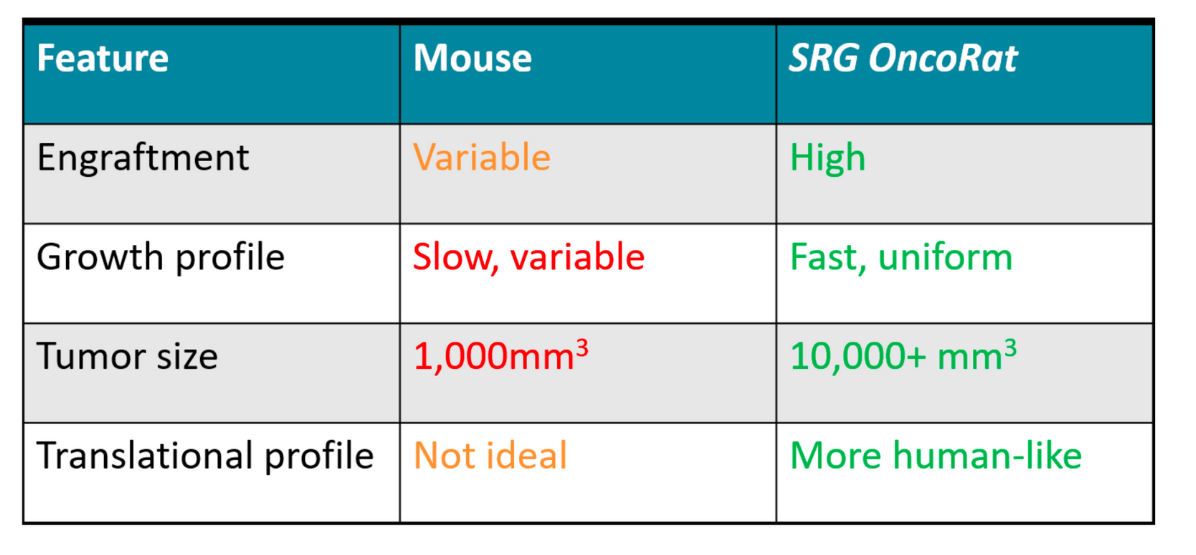
An essential challenge in oncology research is identifying human-relevant model systems. To this end, xenografts are widely used to understand drug effects on human cancer tissues using in vivo models. Historically, that model was a severe combined immunodeficient (scid) or NOD scid gamma (NSG) mouse, but that can have many drawbacks: including low engraftment efficiencies, small tumors, slow growth, and variable tumor morphology. Complicating this picture, toxicology, safety, and pharmacokinetics studies are typically done in rats, where size, physiology, and cross-discipline data collection offer unique advantages for drug development.(3) This means that efficacy studies using xenografts in mice are not as robust as in rats, with the latter providing an overall better translation to humans, particularly given the ability for serial tumor biopsies and blood draws for enhanced pharmacokinetic and pharmacodynamic/ biomarker evaluations.
To take advantage of the rat for oncology research, Hera BioLabs capitalized on advances in gene editing technologies to develop and offer the SRG OncoRat model system. Like NSG mice, the OncoRats lack B, T, and NK cells, which facilitate human tissue engraftment. Xenografts in the OncoRat display vastly improved engraftment efficiencies, tumor size, growth, and tumor morphology across a number of human cancer cell lines and patient-derived tissues that are either not permissive or extremely inefficient in mice. Through the use of the OncoRat, researchers can collect more consistent, higher quality data that provides advantages for predicting success in the clinic. A 2019 publication in Clinical Cancer Research (CCR),(4) highlighted below as a case study, demonstrates the power of the SRG OncoRat in this application.
SRG OncoRat VCaP Prostate Xenograft Tumor Model Facilitates Successful Drug Development
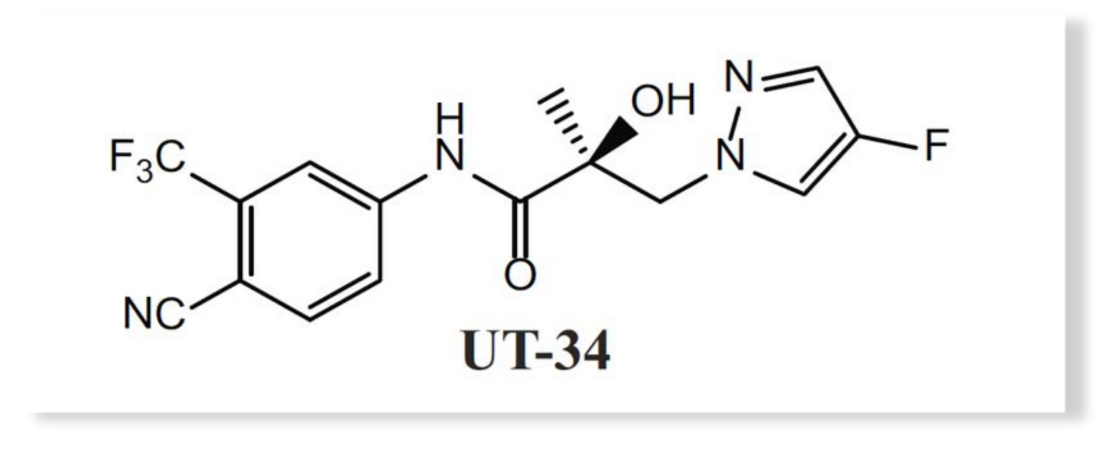
Figure 1: UT-34 structure. Adopted from citation 4.
A team of researchers led by Dr. Ramesh Narayanan at the University of Tennessee sought to develop new therapeutics for castration-resistant prostate cancer (CRPC), targeting androgen receptors (ARs). Since testosterone independent, CRPCs are dependent on AR activity for further growth, conventional approaches, like the FDA-approved enzalutamide, competitively antagonize AR at its ligand binding domain (LBD) to limit its activity. However, mutant LBDs, AR splice variants (AR-SVs), and increased AR expression have been extensively documented in the clinic.(4) These aberrant AR expression profiles in patients correspond with both the development of drug resistance over brief treatment periods and drug response failures. To circumvent this, Dr. Narayanan’s team developed small molecules that instead degrade AR to prevent escape phenotypes. A second-generation molecule, UT-34 (Figure 1), demonstrated good pharmacokinetics and was able to efficiently down regulate AR in wild-type (WT) and drug-resistant cell culture systems that included mutant LBD ARs, AR-SVs, and/or significant AR amplification.
Despite these exciting results, the team struggled to find good disease model systems to confirm the efficacy in vivo. Drug metabolism studies in mouse liver microsomes showed faster metabolism compared to both rat and human liver microsomes (>2-4 fold more stable in the rat and human compared to mice). Pharmacokinetics studies where UT-34 was administered to mice and rats supported these data, indicating rapid clearance in mice but not in rats by 24 hours. Given these pivotal differences between mice and rats, the group decided to perform anticancer efficacy studies in rats to better match the metabolic profile of humans. Ideally, these xenografts would use VCaP cells since they best match the high AR-expression amplification phenotype found in a large percentage of patients with CRPC. However, no VCaP xenograft system existed in rats, stalling the preclinical effort and threatening the ability to move forward.
In time, the research leads contacted Hera BioLabs, inquired about the OncoRat, and a partnership was formed. Hera piloted VCaP xenografts, validated/assessed engraftment efficacy, and delivered rat xenograft models for both parental VCaP and enzalutamide acquired-resistant VCaP cells (MDVR). VCaP engraftment in the OncoRats was 78% more efficient than mice, clearly indicating the capacity of OncoRats to act as xenograft hosts for this cell type.
Castrated OncoRats bearing VCaP or VCaP-MDVR tumors were orally treated with UT-34 or enzalutamide once the tumors reached >2000 mm3. While both treatments reduced tumor size in the parental VCaP, only UT-34 reduced the MDVR xenograft tumors to undetectable levels (Figure 1).3
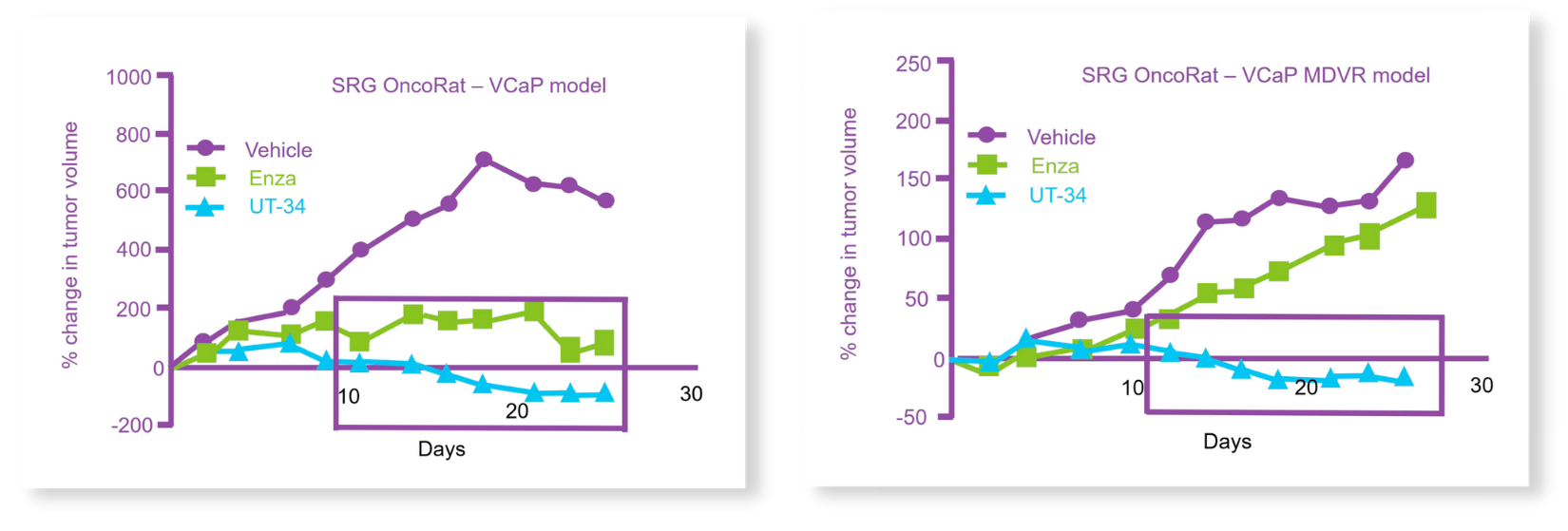
Figure 2: UT-34 regresses the growth of (top) VCaP and (below) enzalutamide-resistant VCaP tumors (MDVR) on OncoRat xenografts. Adopted from citation 4.
Hera BioLabs: Enabling Technologies. Exceptional Services. Data Delivered.
In addition, the authors utilized the OncoRat to test UT-34’s anticancer activity in intact (not castrated) rats, where testosterone levels can outcompete competitive AR-antagonists, such as enzalutamide. Once again, UT-34 inhibited tumor growth in a dose dependent fashion. To date, this is the first time an AR-targeting compound could demonstrate anticancer efficacy on intact rodent xenografts. These two findings, made possible through the OncoRat model, indicate remarkable potential and value for UT-34 that otherwise would have been missed in mouse systems, due to the molecule’s rapid metabolism in mice. Combining both toxicity and efficacy endpoints in the OncoRat helped rescue the preclinical trial outcome and facilitate a potentially valuable anticancer drug into early development.
While this research article discusses a single example of the studies possible with the OncoRat, it represents the diverse potential of OncoRat xenografts. Without it, the program may have ended when the mice models fell short. Hera Biolabs has a number of validated tumor models, and is clearly able to construct new ones (like VCaP) to assist clients in need. In doing so, Hera and its OncoRat have helped a valuable lead compound move closer to treating a costly human disease.
References
1 Wong CH, Siah KW, Lo AW, Estimation of clinical trial success rates and related parameters. Biostatistics, 2019;20(2):273-286.
2 Gamo NJ, Birknow MR, Sullivan D, Kondo MA, et al. Valley of death: A proposal to build a “translational bridge” for the next generation. Neurosci Res, 2017;115:1-4.
3 Iannnaccone PM and Jacob HJ. Rats! Dis Model Mech, 2009;2:206-210.
3 Iannnaccone PM and Jacob HJ. Rats! Dis Model Mech, 2009;2:206-210.
About Hera BioLabs
Hera BioLabs utilizes enabling technologies for preclinical discovery. We provide our clients with exceptional services, preclinical models off-the-shelf and gene editing tools for early drug development applications. Combined with our experienced staff & state- of-the-art facilities we ensure high quality data delivered. Hera’s platform in vivo model, the SRG rat, is a SCID rat on the Sprague- Dawley background harboring a double knockout for the Rag2 and Il2rgamma genes with applications in oncology, immuno- oncology and other areas of pharmacology/toxicology are being developed. Our platform gene editing technology is the piggyBac transposon, with 750 peer-reviewed publications and counting, piggyBac is a highly validated system for cell line engineering and transgenesis.
