Follow as our Grace Walton, PhD reviews validation results from a Sprague-Dawley Rag2/Il2rg double knockout (SRG®) immunodeficient rat. For cancer modeling and drug testing, it demonstrates high efficiency tumor take rates and growth kinetics with different cell lines and PDXs – even those known to be difficult to propagate. Grace also examines novel PDX establishment combined with the use of next generation sequencing (NGS) to aid in the development of more predictive precision cancer models.
Introduction to Hera BioLabs
We offer a suite of proprietary gene editing tools including the piggyBac® non-viral gene delivery transposon system and the Cas-CLOVER™ and TAL-CLOVER™ targeted gene editing systems. We provide these as reagents and we also offer molecular biology services, including vector design, cell line engineering, and animal model generation
We also offer a suite of in vivo work anchored by our SRG™ OncoRat.
The SRG Rat is a highly immunocompromised, superior xenograft host and we’re currently using this rat to develop other models including one with a humanized liver and a humanized immune system. We also offer a number of in vivo contract research services including oncology studies in our srg rat other rats and mice and we provide animal model characterization and validation
What is the SRG OncoRat?
The SRG Rat was developed on the Sprague-Dawley background. It’s a double knockout for il-2 receptor gamma and rag2. It’s a SCID rat with enhanced immunodeficiency because it lacks B-cells and circulating NK-cells as well as T-cells, which in turn enables enhanced engraftment rates for PDXs and CDXs as well as enhanced tumor growth. Moreover, since it supports larger tumors and larger animals, we can perform serial tumor biopsies and serial blood draws, and we can obtain more tissue at necropsy allowing us to combine pharmacokinetic, biomarker toxicity, and drug efficacy studies.
A Prostate Cancer Study in the SRG Rat
The data in Figure 1 were obtained following subcutaneous inoculation of the same number of VCaP cells into the SRG Rats and NSG mice. The VCaP growth is far more robust in the SRG Rat than it is in the mice and we often obtain roughly 10 times the tumor volume from SRG Rats compared to NSG mice.
Importantly, we can proceed to efficacy studies faster than we would while using mice since we do not have to passage as often for PDX’s or for cell lines.
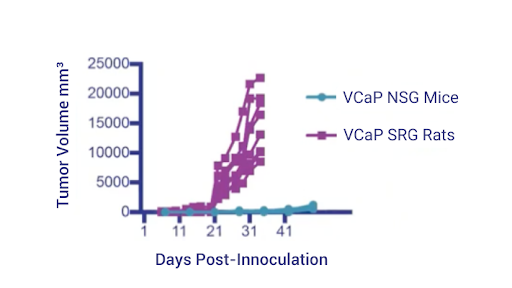
Figure 1: VCaP tumor growth in SRG Rat vs NSG Mouse
For precision medicine, SRG Rats also offer the advantage that they accelerate the timeline from a patient biopsy to efficacy testing in animals.
The VCaP tumor in the SRG Rat maintains androgen receptor expression and prostate-specific antigen expression after passaging. In Figure 2, see an example of how we’re able to leverage serial blood draws.
As the tumors were growing, these animals had blood draws weekly and we measured PSA in the serum. We were able to see tight correlation between serum PSA and tumor volumes as the tumors were growing.
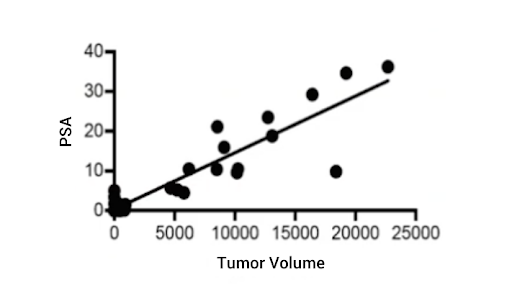
Figure 2: VCaP Tumor Serum PSA
A case study – PDX establishment in the SRG Rat
One of our ongoing efforts is to establish new PDX models in the SRG Rat. For this study, tumor tissue was taken from a patient biopsy and used to establish a pdx in the srg rat and we were able to provide actionable data back to the clinic to inform treatment decisions
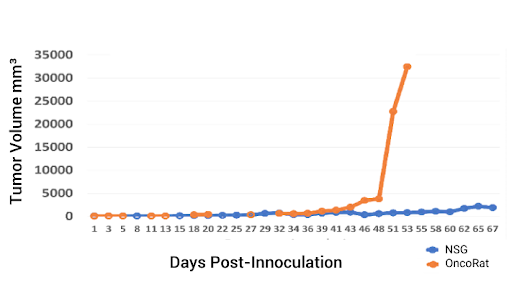
Figure 3: PDX tumor kinetics in the SRG Rat versus the NSG Mouse.
Working with the University of Kentucky Markey Cancer Center, we obtained a sample of non-small cell lung carcinoma biopsy from a middle aged white male, and we used this to develop PDX 3010. We first inoculated the same amount of tissue – eight cubic millimeters – into NSG mice and SRG Rats. Data clearly show that both the time to establishment and the tumor growth were much faster in the rats (Figure 3).
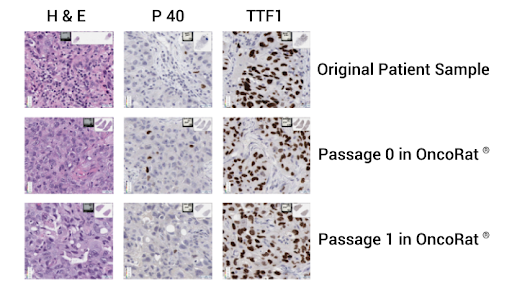
Figure 4: NSCLC PDX patient 3010 histology and genetic mutation identification.
Further, from P0 on, we can obtain enough tissue to perform gene sequencing and histology. In this case, we ran histology on tumors taken from the rats at each passage and we show that they remain positive for TTF1 and negative for P40 – indicating that they are indeed the adenocarcinoma and not a squamous cell carcinoma (Figure 4).
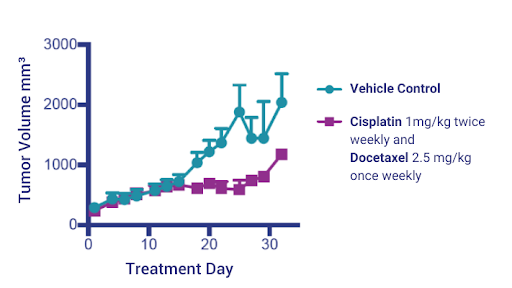
Figure 5: NSCLC PDX Model 3010 tumor volumes treated with Cisplatin and Docetaxel charted over time.
After establishing the tumor in the SRG Rats, we then returned to NSG mice for efficacy studies. We proceeded to test standard-of-care treatment against PDX 3010 with Cisplatin and Docetaxel, and observed a moderate response (Figure 6).
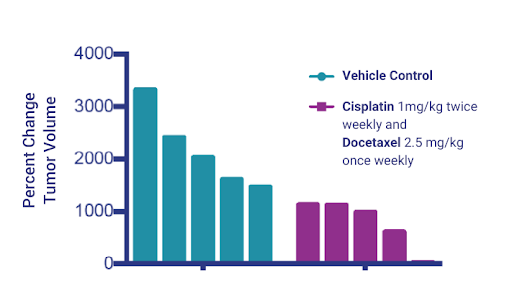
Figure 6: Changes in volumes of tumors treated with Cisplatin and Docetaxel.
Since we didn’t see a sufficient response to Cisplatin and Docetaxel, we proceeded to perform genetic characterization of the tumors in an effort to identify efficacious treatment strategies.
Our collaborators in Gautam Narla’s lab at the University of Michigan performed targeted exome sequencing and identified a mutation in the MET gene which was a tyrosine to histidine substitution at amino acid 1248. This was interesting because substitutions at this tyrosine have been reported in papillary renal carcinomas and are believed to be likely pathogenic.
Due to evidence that the mutation was pathogenic, we wanted to know whether MET inhibitors would inhibit PDX 3010 tumor growth. Dr. Narla found a 2017 publication, which showed that the 1248 tyrosine mutation in non-small cell lung cancer did in fact lead to the resistance of type 1 meta inhibitors such as crizotinib but these same tumors were sensitive to the type II met inhibitor Cabozantinib
Based on these data, Dr, Narla and our team hypothesized that PDX 3010 would be sensitive to the type II inhibitor Cabozantinib.
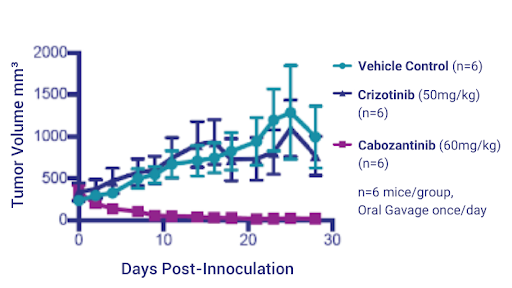
Figure 7: NSCLC PDX Model 3010 tumor volumes treated with Crizotinib and Cabozantinib charted over time.
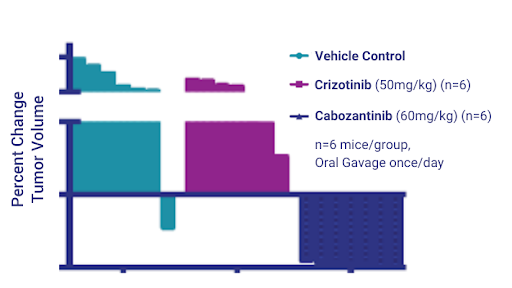
Figure 8: Change in NSCLC PDX Model 3010 tumor volumes treated with Crizotinib and Cabozantinib.
In the NSG mice, we proceeded to test the two drugs side by side. After dosing for 28 days, we saw no effect of Crizotinib but we saw nearly complete tumor regression with Cabozantinib and, as with the previous platinum study, the histogram on the right hand side shows percent change in tumor volume in individual mice (Figures 7 and 8).
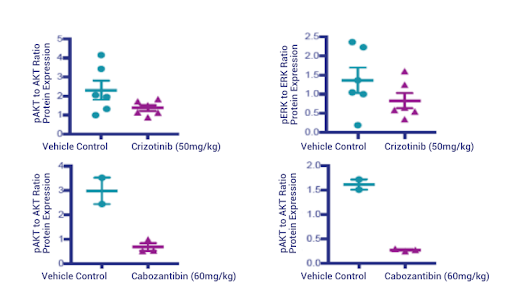
Figure 9: Western Blot quantification of phospho- and total AKT and phospho- and total ERK.
To verify the molecular mechanism of tumor regression following cabozantinib treatment, we performed western blots for phospho- and total AKT and phospho- and total ERK. Our data show that Crizotinib failed to really completely suppress phospho-AKT (pAKT) and phospho-ERK (pERK) while Cabozantinib completely obliterated the phospho- signal (Figure 9). Further, the IHC underscored the western blot findings; Crizotinib does not suppress pAKT and pERK while Cabozantinib makes them almost undetectable.
Conclusion
So, in conclusion, the SRG OncoRat can vastly shorten the timeline to actually be able to provide data back to clinicians. A fantastic host for establishing PDXs, this model enables fast tumor growth.
The SRG Rat also succeeds with difficult cancer cells that are hard to grow in mice like PDX 3010. We were able to identify a pathogenic mutation and communicate that back to the clinical oncologist, but this is just the beginning. We are continuing to work with the SRG rat to continue to improve precision medicine.
We have ample resources for scientists in this space, including PDX cell lines established from lung and ovarian cancers, PDX establishment services for new or custom PDXs, and custom gene editing including cell line engineering and bespoke animal model development.
Schedule a call with us today if this could help your CDX or PDX study.
