About VCaP
The VCaP cell line, derived from a vertebral metastasis of a 57-year-old Caucasian male with prostate cancer, offers valuable insights into cell signaling and molecular biology in the context of prostate cancer. These epithelial-like cells are grown as adherent monolayers in vitro, providing a controlled experimental system for studying various aspects of prostate cancer biology. The VCaP cell line expresses androgen receptor (AR) and prostate-specific antigen (PSA), both of which are characteristic markers for prostate cancer.
Notably, VCaP cells express both the wild-type AR and a gene fusion between AR and the ERG transcription factor. This gene fusion event has been implicated in prostate cancer progression and provides an opportunity to investigate the molecular mechanisms associated with AR signaling and its impact on tumor development and progression. VCaP cells have demonstrated susceptibility to enzalutamide, a potent anti-androgen therapy used in the treatment of prostate cancer. This feature makes the VCaP cell line particularly valuable for efficacy studies of enzalutamide or related compounds on cell growth, viability, and signaling pathways associated with AR inhibition.
The VCaP cell line can serve as a positive control for AR translocation assays, enabling researchers to assess the cellular localization and activation of the AR protein in response to various stimuli or drug treatments. This feature provides insight into the dynamic nature of AR signaling and its role in prostate cancer progression.
VCaP Tumor Kinetics in the SRG™ Rat
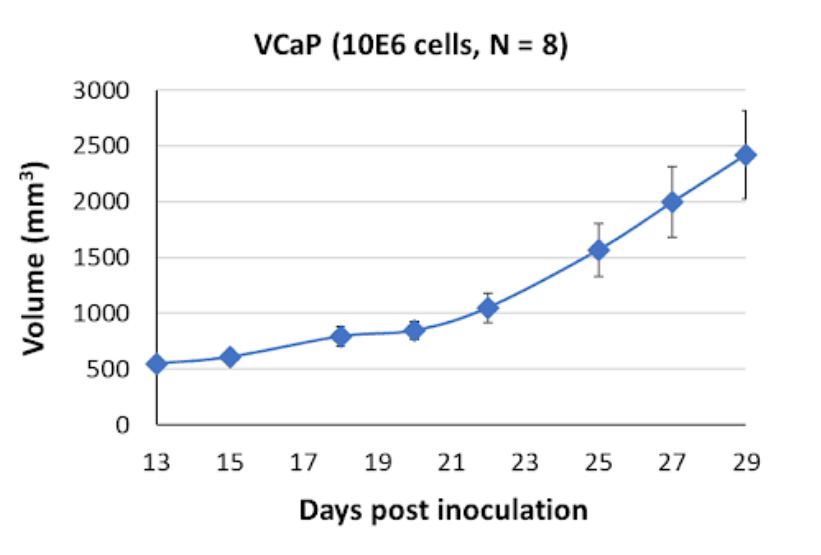
When implanted in SRG rats, VCaP cells give rise to soft and vascularized tumors. This tumor phenotype reflects the growth characteristics and angiogenic potential of VCaP cells. This in vivo model can be used to investigate the tumor microenvironment, tumor-host interactions, and potential therapeutic interventions targeting angiogenesis.
Products & Services
Xenograft Efficacy Studies
Includes collection of blood, tissues & tumor for ADME, PK/PD and analysis.
(Bi)weekly Tumor Sampling
Via fine needle aspiration (FNA). For longitudinal evaluation of drug exposure, histology and gene expression.
OncoRats
Cutting edge models optimized for engraftment.
Get help with your research by scheduling a call with Hera.
Interested in our full list of Validated Tumor Models?
References
- https://doi.org/10.1038/s41598-021-81164-0 10.3389/fonc.2018.00180

