About OCI-AML2
The OCI-AML2 cell line is derived from the bone marrow of a 60-year-old woman diagnosed with acute myeloid leukemia (AML). It was established in 1988 by T. Tanaka et al. OCI-AML2 cells exhibit a myeloblast-like morphology and grow in suspension culture. This cell line has been instrumental in advancing our understanding of AML pathobiology, particularly in relation to specific cell signaling pathways. One of the key genetic features of OCI-AML2 cells is the presence of a t(8;21)(q22;q22) translocation, which is characteristic of 15-20% of AML cases. This translocation event involves the fusion of two genes: the runt-related transcription factor 1 (RUNX1) gene and the core-binding factor beta (CBFb) gene.
RUNX1 is a critical DNA-binding element of the core-binding factor (CBF) transcription complex, which plays a crucial role in hematopoietic cell development and regulation of gene expression. The fusion of RUNX1 with CBFb results in the formation of a chimeric protein that dysregulates normal hematopoiesis, contributing to the development of AML. The t(8;21) translocation in OCI-AML2 cells has been associated with positive patient outcomes, suggesting a distinct molecular subtype of AML. This fusion protein leads to enhanced binding affinity of RUNX1 to its target genes, altering gene expression patterns and promoting leukemogenesis. Understanding the specific downstream signaling pathways affected by the RUNX1-CBFb fusion protein in OCI-AML2 cells is critical for unraveling the molecular mechanisms underlying AML development and identifying potential therapeutic targets. Historic data suggests that knockout or disruption of the RUNX1-CBFb fusion protein in animal models leads to loss of definitive hematopoiesis and impedes leukemogenesis. This highlights the crucial role of the RUNX1-CBFb fusion protein in driving aberrant hematopoietic cell growth and differentiation observed in AML.
The OCI-AML2 cell line serves as an excellent model system to study the molecular consequences of this translocation and its impact on leukemogenesis. In terms of therapeutic relevance, OCI-AML2 cells have shown sensitivity to histone deacetylase inhibitors (HDIs). HDIs target enzymes involved in histone deacetylation, leading to chromatin remodeling and modulation of gene expression. In OCI-AML2 cells, treatment with HDIs has demonstrated promising results in inhibiting cell proliferation and promoting differentiation, making them a valuable model system for the development and testing of HDI-based therapies for AML.
When xenografted into animal models, OCI-AML2 cells produce soft, flat tumors. The tumor phenotype observed in vivo reflects the characteristics of OCI-AML2 cells, such as their suspension growth and myeloblast-like morphology. These xenograft models provide a valuable platform for studying the behavior of AML cells in a more complex in vivo environment, including tumor growth dynamics, response to therapy, and the role of the microenvironment in leukemogenesis.
OCI-AML2 Tumor Kinetics in the SRG™ Rat
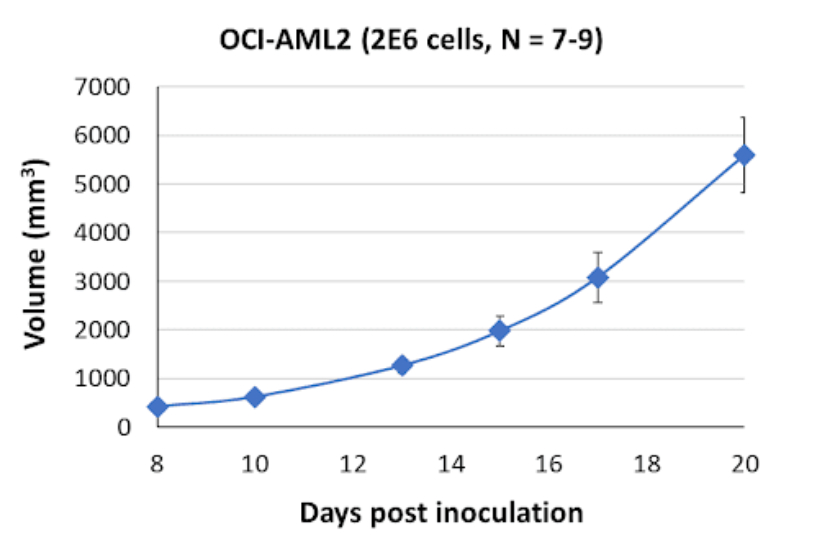
Here is the growth curve for this cell line’s tumors in Hera’s SRG Rat.
Products & Services
Xenograft Efficacy Studies
Includes collection of blood, tissues & tumor for ADME, PK/PD and analysis.
(Bi)weekly Tumor Sampling
Via fine needle aspiration (FNA). For longitudinal evaluation of drug exposure, histology and gene expression.
OncoRats
Cutting edge models optimized for engraftment.
Get help with your research by scheduling a call with Hera.
References
- 10.1182/blood-2016-10-687830
- 10.1182/blood.2020007747
- https://www.ncbi.nlm.nih.gov/gene?Cmd=DetailsSearch&Term=865
References MLA
- “CBFB Core-Binding Factor Subunit Beta [Homo Sapiens (Human)] – Gene – NCBI.” National Center for Biotechnology Information, June 2023, www.ncbi.nlm.nih.gov/gene?Cmd=DetailsSearch&Term=865.
- Sood, Raman, et al. “Role of RUNX1 in Hematological Malignancies.” Ashpublications.Org, 13 Apr. 2017, ashpublications.org/blood/article/129/15/2070/36427/Role-of-RUNX1-in-hematological-malignancies.
- Zhen, Tao, et al. “RUNX1 and CBFβ-SMMHC Transactivate Target Genes Together in Abnormal Myeloid Progenitors for Leukemia Development.” Ashpublications, 19 Nov. 2020, ashpublications.org/blood/article/136/21/2373/463758/RUNX1-and-CBF-SMMHC-transactivate-target-genes.

