About NCI-H1734
The NCI-H1734 cell line is derived from human epithelial non-small-cell lung carcinoma and was obtained from the lung tissue of a 56-year-old female patient. It was established in September 1987 and has since played a crucial role in advancing our understanding of lung cancer biology. NCI-H1734 cells are cultured optimally in RPMI medium 1640 supplemented with 10% fetal bovine serum (FBS).
This cell line exhibits several important genetic alterations that contribute to its oncogenic phenotype and therapeutic responsiveness. NCI-H1734 cells feature the L858R mutation in exon 21 of the epidermal growth factor receptor (EGFR). This mutation results in a single amino acid substitution in the EGFR protein, leading to its constitutive activation. The EGFR pathway plays a crucial role in cell growth, survival, and proliferation, and its dysregulation is frequently observed in non-small-cell lung carcinoma. The presence of the L858R mutation in NCI-H1734 cells makes this cell line a valuable model for studying the role of EGFR in lung cancer oncogenesis and investigating therapeutic strategies targeting EGFR signaling.
In addition, NCI-H1734 cells harbor a KRAS G12C mutation. KRAS is a member of the RAS family of oncogenes and plays a central role in cell signaling networks that regulate proliferation, survival, and differentiation. The specific G12C mutation in KRAS observed in NCI-H1734 cells results in constitutive activation of downstream signaling pathways, contributing to cellular transformation and tumor progression. The presence of both EGFR and KRAS mutations in NCI-H1734 cells underscores the complexity and heterogeneity of non-small-cell lung carcinoma and provides an excellent model for studying the interplay between these key oncogenic drivers.
Furthermore, NCI-H1734 cells exhibit overexpression of tyrosine-protein kinase Met (c-Met). c-Met is a receptor tyrosine kinase involved in various cellular processes, including cell growth, survival, motility, and invasion. Overexpression of c-Met in NCI-H1734 cells indicates its potential contribution to the malignant phenotype of this cell line and its involvement in promoting lung cancer progression. Drug sensitivity studies have revealed that NCI-H1734 cells are primarily sensitive to DNA alkylating agents, which cause structural damage to DNA.
NCI-H1734 cells have shown sensitivity to XIAP (X-linked inhibitor of apoptosis protein) targeted agents. XIAP is an anti-apoptotic protein that confers resistance to cell death pathways, and targeting XIAP has emerged as a potential therapeutic strategy for enhancing the efficacy of anticancer treatments. Conversely, NCI-H1734 cells have exhibited resistance to cediranib, a tyrosine kinase inhibitor targeting vascular endothelial growth factor (VEGF) receptors involved in angiogenesis.
NCI-H1734 Tumor Kinetics in the SRG™ Rat
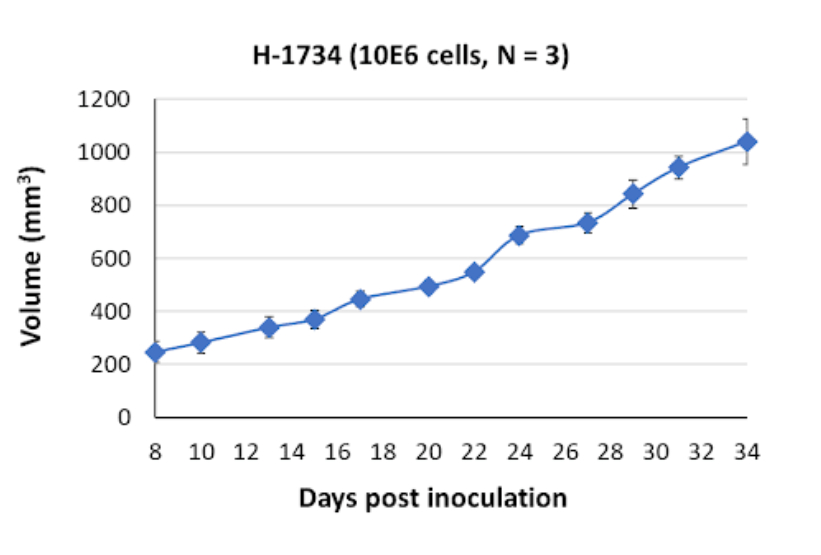
In vivo, NCI-H1734 cells produce firm, vascularized tumors that tend to exhibit slow growth. This tumor phenotype in vivo reflects the characteristics of NCI-H1734 cells and provides a platform for studying tumor growth dynamics, angiogenesis, and therapeutic responses in a more complex physiological context.
Products & Services
Xenograft Efficacy Studies
Includes collection of blood, tissues & tumor for ADME, PK/PD and analysis.
(Bi)weekly Tumor Sampling
Via fine needle aspiration (FNA). For longitudinal evaluation of drug exposure, histology and gene expression.
OncoRats
Cutting edge models optimized for engraftment.
Get help with your research by scheduling a call with Hera.
References (MLA):
- Addeo, Alfredo, et al. “Kras G12C Mutations in NSCLC: From Target to Resistance.” MDPI, 21 May 2021, www.mdpi.com/2072-6694/13/11/2541.
- Fong, Jason T., et al. “Alternative Signaling Pathways as Potential Therapeutic Targets for Overcoming EGFR and C-MET Inhibitor Resistance in Non-Small Cell Lung Cancer.” PLOS ONE, 4 Nov. 2013, journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0078398.
- Hong, Weiwei, et al. “Prognostic Value of EGFR 19‑del and 21‑L858R Mutations in Patients with Non‑small Cell Lung Cancer.” Oncology Letters, 1 Aug. 2019, www.spandidos-publications.com/10.3892/ol.2019.10715.
- Latour, Sylvain, and Claire Aguilar. “XIAP Deficiency Syndrome in Humans.” Seminars in Cell & Developmental Biology, 7 Feb. 2015, www.sciencedirect.com/science/article/abs/pii/S1084952115000270?via%3Dihub.

