About MX-1
The MX-1 cell line is used to study the intricate molecular mechanisms underlying breast cancer, specifically triple-negative breast cancer (TNBC). Derived from a 29-year-old Caucasian female with metastatic breast cancer, MX-1 cells represent an aggressive and clinically challenging subtype of breast cancer. This cancer cell line is characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression.
In vitro, MX-1 cells exhibit an adherent monolayer growth pattern, morphologically resembling breast epithelial cells. With a doubling time of 36 hours, these cells possess a moderate proliferation rate, allowing for efficient experimentation and investigation of cell signaling and molecular events. MX-1 cells have a triple-negative phenotype, which significantly impacts their response to therapeutic interventions. The absence of ER, PR, and HER2 receptors makes MX-1 cells refractory to targeted therapies commonly used in other breast cancer subtypes. However, MX-1 cells exhibit sensitivity to various chemotherapeutic agents, making this line an important model for studying the efficacy and development of alternative therapeutic strategies for TNBC.
MX-1 Tumor Kinetics in the SRG™ Rat
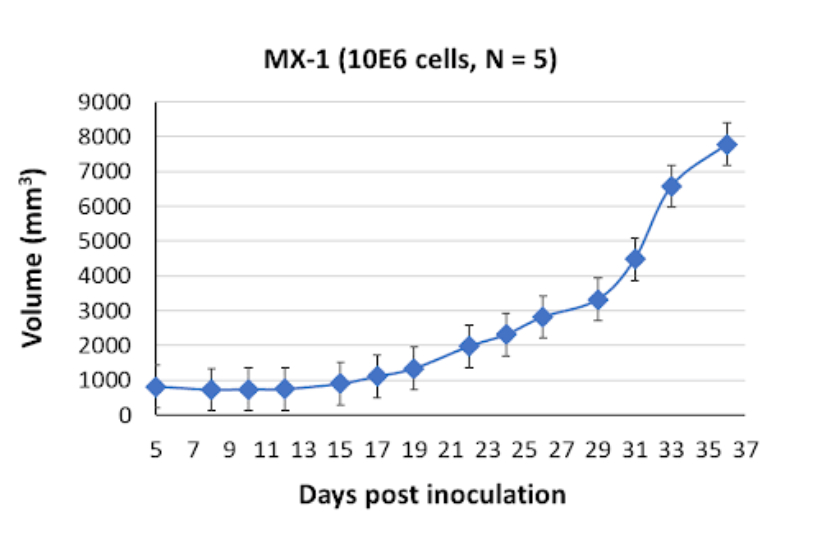
Investigating the molecular determinants responsible for chemosensitivity in MX-1 cells can shed light on potential therapeutic targets and guide the development of more effective treatment regimens for TNBC patients. Researchers can investigate the molecular alterations induced by these agents, elucidating the signaling pathways and molecular targets involved in mediating their cytotoxic effects on MX-1 cells. These studies can contribute to the identification of biomarkers and predictive signatures that aid in patient stratification and personalized medicine approaches for TNBC.
Products & Services
Xenograft Efficacy Studies
Includes collection of blood, tissues & tumor for ADME, PK/PD and analysis.
(Bi)weekly Tumor Sampling
Via fine needle aspiration (FNA). For longitudinal evaluation of drug exposure, histology and gene expression.
OncoRats
Cutting edge models optimized for engraftment.
Get help with your research by scheduling a call with Hera.
References (MLA):
- Li, Yun, et al. “Recent Advances in Therapeutic Strategies for Triple-Negative Breast Cancer .” BioMed Central, 29 Aug. 2022, jhoonline.biomedcentral.com/articles/10.1186/s13045-022-01341-0.
- Onitilo, Adedayo, et al. “Breast Cancer Subtypes Based on ER/PR and Her2 Expression: Comparison of Clinicopathologic Features and Survival.” Clinical Medicine & Research, 1 June 2009, www.clinmedres.org/content/7/1-2/4.
- Rubin, I, and Y Yarden. “The Basic Biology of HER2.” Pubmed, 2001, pubmed.ncbi.nlm.nih.gov/11521719/.

