About MCF7
The MCF-7 cell line, derived from a tumor of a 69-year-old woman with metastatic breast cancer, has played a pivotal role in advancing our understanding of breast cancer biology. Established by Herbert Soule and colleagues in 1970, MCF-7 cells exhibit epithelial characteristics and grow as a monolayer in vitro. MCF-7 cells also possess the capability to form domes, a characteristic morphology of differentiated mammary epithelium. This dome-forming ability is indicative of the cell line’s retained functional attributes of mammary glandular architecture. Retaining several characteristics of differentiated mammary epithelium, MCF-7 cells can process estradiol via cytoplasmic estrogen receptors, which reflects their capacity to respond to hormonal stimulation.
MCF-7 cells express hormone receptors, including estrogen receptor (ER) and progesterone receptor (PR). This receptor-positive status allows MCF-7 cells to respond to estrogen stimulation, making them a valuable model for studying estrogen receptor signaling and anticancer hormone therapies, such as the widely used drug tamoxifen.
In addition to hormone receptor expression, MCF-7 cells produce matrix metalloproteinases (MMPs) and urokinase plasminogen activators (uPAs). MMPs are cytoplasmic enzymes involved in remodeling the extracellular matrix, which plays a critical role in tumor invasion and metastasis. uPAs, on the other hand, are involved in the activation of plasminogen, leading to the breakdown of extracellular matrix components. The production of MMPs and uPAs by MCF-7 cells reflects their invasive potential and provides a platform for studying the mechanisms underlying tumorigenicity and metastasis in breast cancer.
Researchers have utilized MCF-7 cells to assay estrogen signaling pathways and hormone-dependent gene regulation. The ability to manipulate hormone levels and observe the resulting cellular responses in MCF-7 cells provides valuable insights into the molecular mechanisms involved in hormone-dependent breast cancer growth. Furthermore, the MCF-7 cell line has been instrumental in studying drug resistance mechanisms in breast cancer. Additionally, MCF-7 cells express the WNT7B oncogene, which plays a crucial role in various cell cycle processes, including cell proliferation, differentiation, and tissue development. The presence of the WNT7B oncogene in MCF-7 cells adds another layer of complexity to their molecular profile. Investigations into the role of WNT7B in MCF-7 cells can shed light on its contribution to breast cancer development, progression, and potential therapeutic targets.
MCF7 Tumor Kinetics in the SRG™ Rat
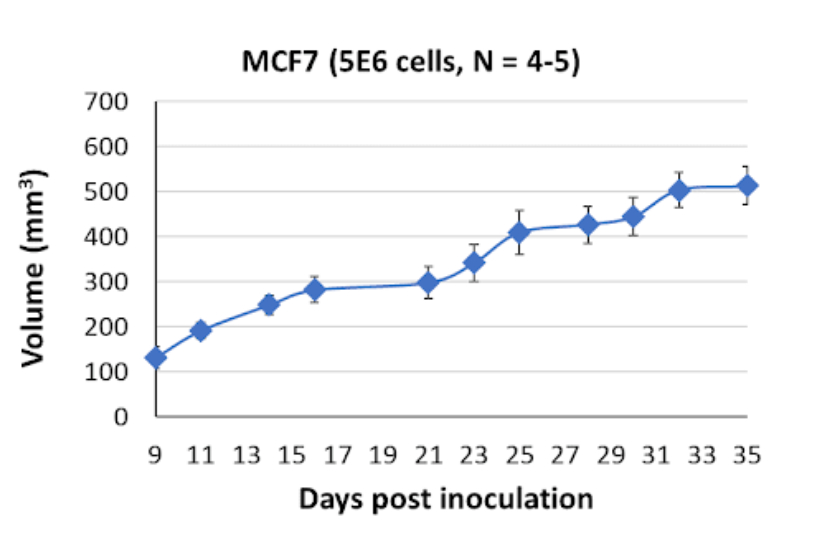
In vivo, MCF7 cells form necrotic and undefined, fluid-filled tumors when implanted in the SRG rat. This tumor phenotype highlights the aggressive cell proliferation of MCF-7 cells and provides a platform for studying tumor growth, angiogenesis, and response to therapeutic interventions in vivo.
Products & Services
Xenograft Efficacy Studies
Includes collection of blood, tissues & tumor for ADME, PK/PD and analysis.
(Bi)weekly Tumor Sampling
Via fine needle aspiration (FNA). For longitudinal evaluation of drug exposure, histology and gene expression.
OncoRats
Cutting edge models optimized for engraftment.
Get help with your research by scheduling a call with Hera.
References
- Kirikoshi H, Katoh M. Expression of WNT7A in human normal tissues and cancer, and regulation of WNT7A and WNT7B in human cancer. Int J Oncol. 2002 Oct;21(4):895-900. PMID: 12239632. https://doi.org/10.1002/dvdy.24554

