About HCT-116
The HCT-116 cell line, established in 1988 from a tumor sample of a 44-year-old male patient with colon cancer, has been instrumental in advancing our understanding of colorectal carcinoma. These cells exhibit an adherent growth pattern, appearing as polygonal epithelial cells that form a monolayer in vitro. The HCT-116 cell line carries a dysfunctional DNA mismatch repair pathway, which arises from a mutation in codon 13 of the Ras proto-oncogene. The DNA mismatch repair pathway plays a critical role in correcting errors that occur during DNA replication. In HCT-116 cells, this dysfunction leads to rapid accumulation of genetic mutations and a higher propensity for genomic instability. This feature makes the HCT-116 cell line an ideal model for studying the molecular mechanisms underlying DNA damage and repair processes, as well as for evaluating the efficacy of DNA-damaging therapies.
HCT-116 cells can grow in an anchorage-independent manner, which is a hallmark of aggressive cancer cell lines. This property allows cells to form colonies in suspension, reflecting their ability to overcome the normal anchorage-dependent growth control mechanisms. Studying the anchorage-independent growth of HCT-116 cells provides insights into the mechanisms involved in tumor progression and metastasis. HCT-116 cell derived xenografts overexpress matrix metalloproteinases, urokinase plasminogen activators, and vascular endothelial growth factor. These molecular markers are associated with tumor invasion, angiogenesis, and metastasis. The presence of these markers in HCT-116-derived xenografts accurately recapitulates the aggressive nature of colorectal carcinoma and provides a platform for studying the molecular pathways involved in tumor progression.
The HCT-116 cell line is particularly valuable in molecular studies and efficacy assessments of DNA-damaging therapies. The dysfunctional DNA mismatch repair pathway in HCT-116 cells increases their sensitivity to DNA-damaging agents, making them a suitable model for evaluating the effectiveness of such therapies. Additionally, the HCT-116 cell line serves as a positive control in PCR mutation assays due to its known mutation in the Ras proto-oncogene. This feature enables researchers to assess the accuracy and sensitivity of mutation detection methods.
Therapeutic efficacy studies in HCT-116 cells have demonstrated a sensitivity to agents that target ERK1/ERK2, such as ulixertinib. ERK1/ERK2 are key components of the mitogen-activated protein kinase (MAPK) pathway, which plays a crucial role in cell proliferation, survival, and differentiation. Targeting the ERK1/ERK2 pathway has shown promise as a therapeutic strategy in colorectal carcinoma, and HCT-116 cells provide a valuable model for investigating the efficacy of such targeted agents.
HCT116 Tumor Kinetics in the SRG™ Rat
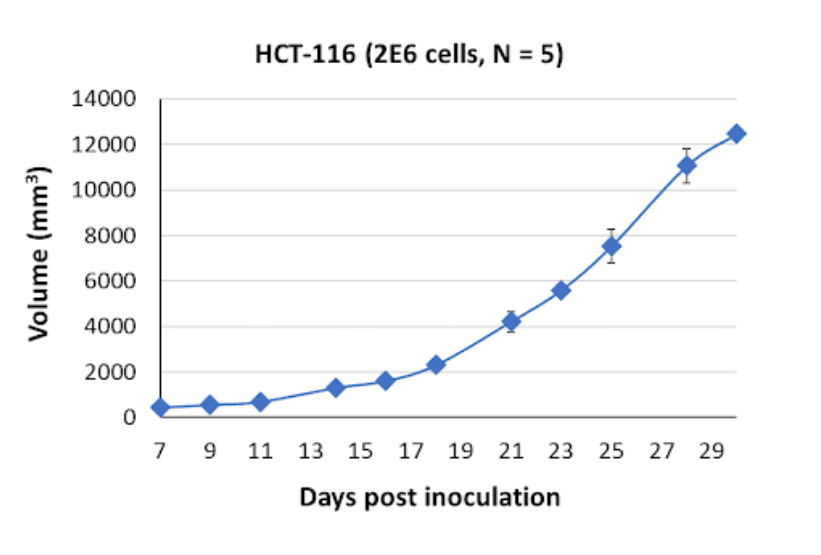
In the SRG rat model, tumors derived from HCT-116 cells exhibit firm consistency but also display necrotic and fluid-filled characteristics. This tumor phenotype reflects the aggressive nature of colorectal carcinoma and provides a reliable model for studying tumor growth, response to therapies, and the interaction between tumor cells and the surrounding microenvironment.
Products & Services
Xenograft Efficacy Studies
Includes collection of blood, tissues & tumor for ADME, PK/PD and analysis.
(Bi)weekly Tumor Sampling
Via fine needle aspiration (FNA). For longitudinal evaluation of drug exposure, histology and gene expression.
OncoRats
Cutting edge models optimized for engraftment.
Get help with your research by scheduling a call with Hera.
References
- https://doi.org/10.1016/j.jss.2007.04.021
- https://doi.org/10.3892/etm.2020.8454
References MLA
- Guo, Yan‑Jun, et al. “Erk/MAPK Signalling Pathway and Tumorigenesis (Review).” Experimental and Therapeutic Medicine, 1 Mar. 2020, www.spandidos-publications.com/10.3892/etm.2020.8454.
- Rajput, Ashwani, et al. “Characterization of HCT116 Human Colon Cancer Cells in an Orthotopic …” Journal of Surgical Research, 2004, www.journalofsurgicalresearch.com/article/S0022-4804(07)00278-8/fulltext.

