About HCC-95
HCC-95, a human non-small cell lung cancer (NSCLC) cell line, serves as a valuable resource for postdoctoral researchers studying lung cancer and its underlying molecular mechanisms. Established in 1986 from a primary lung tumor, HCC-95 cells exhibit an epithelial morphology and grow in adherent culture conditions. The unique characteristics of HCC-95 cells contribute to their high tumorigenicity, making them an ideal model for investigating the intricate processes driving NSCLC progression.
The tumorigenic potential of HCC-95 cells can be attributed, in part, to specific genetic alterations. Deletions in the TP53 and cyclin-dependent kinase inhibitor 2A (CDKN2A) genes play a pivotal role in the tumorigenicity of HCC-95 cells. The CDKN2A gene encodes two important CDK4 inhibitors, p14 and p16. p14 acts as an alternative activator of the p53 pathway, providing an additional layer of tumor suppression. The loss or dysfunction of p14 diminishes the robustness of the TP53 tumor suppression system, potentially contributing to uncontrolled cell growth and genomic instability. p16 is a key component of cell cycle regulation that prevents the initiation of the S-phase, ensuring proper cell division. Dysregulation of p16 disrupts this checkpoint and can result in aberrant cell proliferation and tumorigenesis.
In addition, HCC-95 cells exhibit overexpression of the epidermal growth factor receptor (EGFR). EGFR is a receptor tyrosine kinase that plays a crucial role in cell signaling, including the activation of the PI3K pathway. Dysregulated EGFR signaling has been implicated in various aspects of cancer development, including increased cell proliferation and survival. The overexpression of EGFR in HCC-95 cells contributes to the dysregulation of the PI3K pathway, potentially leading to uncontrolled cell growth and survival.
HCC-95 cells are of pharmacological interest due to their resistance to a broad spectrum of chemotherapeutic agents. This resistance poses a significant challenge in the treatment of NSCLC patients and highlights the need for alternative therapeutic strategies. By studying HCC-95 cells, researchers can investigate the underlying mechanisms of this resistance, including alterations in drug efflux pumps, DNA repair pathways, and cell survival signaling cascades. Such studies can provide crucial insights into the development of targeted therapies that specifically address the resistance mechanisms exhibited by HCC-95 cells and similar drug-resistant NSCLC tumors.
HCC-95 Tumor Kinetics in the SRG™ Rat
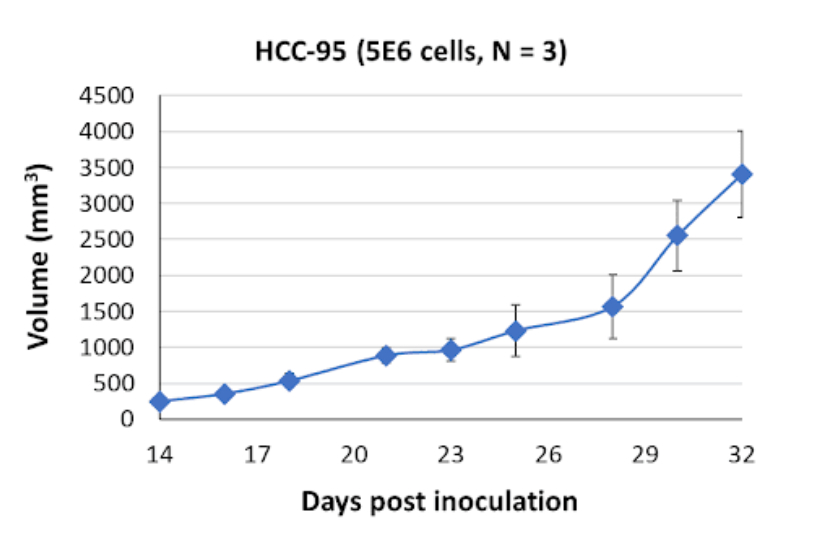
In xenograft models using SRG rats, HCC-95 cells form cystic, fluid-filled tumors. These tumor characteristics in vivo mimic certain aspects of clinical NSCLC, including the presence of cystic lesions. Researchers can exploit these xenograft models to investigate tumor microenvironment interactions, angiogenesis, and potential therapeutic interventions. Understanding the factors contributing to the cystic phenotype of HCC-95 tumors can provide valuable insights into the complex interplay between cancer cells and their surrounding environment.
Products & Services
Xenograft Efficacy Studies
Includes collection of blood, tissues & tumor for ADME, PK/PD and analysis.
(Bi)weekly Tumor Sampling
Via fine needle aspiration (FNA). For longitudinal evaluation of drug exposure, histology and gene expression.
OncoRats
Cutting edge models optimized for engraftment.
Get help with your research by scheduling a call with Hera.
References (MLA):
- “CDKN2A Cyclin Dependent Kinase Inhibitor 2A [Homo Sapiens (Human)] – Gene – NCBI.” National Center for Biotechnology Information, June 2023, www.ncbi.nlm.nih.gov/gene/1029.
- Fong, Jason T., et al. “Alternative Signaling Pathways as Potential Therapeutic Targets for Overcoming EGFR and C-MET Inhibitor Resistance in Non-Small Cell Lung Cancer.” PLOS ONE, Nov. 2013, journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0078398.
- Zhao, Hua, et al. “Generation and Multiomic Profiling of a TP53/CDKN2A Double-Knockout Gastroesophageal Junction Organoid Model.” Science, Nov. 2022, www.science.org/doi/10.1126/scitranslmed.abq6146.
