Xenograft tumors from human cancer cell lines in immunocompromised mice are a staple of pre-clinical oncology research. However, tumor size limits, small blood volume, and minimal toxicology capabilities are significant challenges when using mice as an animal model. Hera BioLabs is proud to offer improved preclinical study opportunities using the SRG rat (OncoRat®), with tumor volumes growing up to 10,000+ mm³ or ten times the size of a mouse xenograft tumor. With larger tumor sizes, serial biopsy by fine needle aspirate (FNA) analysis can be completed to study tumor biology over time, all in the same animal and without inhibiting tumor growth.
The OncoRat® – A Perfect Complement For FNA Analysis
Similar to the NSG mouse, the OncoRat® lacks B, T, and NK cells, enabling efficient human tumor establishment. Extensive xenograft studies in the OncoRat® have shown improved engraftment efficiencies, tumor growth, size, and morphology compared to similar mouse models. Using the OncoRat®, toxicology, safety and pharmacokinetic/pharmacodynamic studies can be done in the same animal, while allowing for increased blood draws and larger tumor volumes that aid analysis after excision.
FNA Analysis Can Make Your Oncologic Study Data More Robust
In addition to post-tumor excision benefits, the large tumor size supported by the OncoRat® is ideal for serial tumor sampling while the animal is still undergoing treatment. Adding FNA data to your research will increase analysis opportunities for protein biomarkers, histology analysis, immune cell infiltration, RNA biomarkers, and RNA-seq to discover novel gene expression changes as the tumor is treated. These FNA analysis tools enable researchers to study tumor biology without sacrificing the animal, therefore decreasing costs and the number of animals associated with your study.
Moreover, serial FNA sampling will deliver the most complete picture of changes occurring inside the tumor as they are treated, not from a sole timepoint at the end of the study. Hera Biolabs has shown that up to two FNA samples can be taken weekly without affecting tumor growth (Figure 1), so one sample can go to histology and the other towards RNA/protein analysis!
Flexible Options for Your Unique Study
Hera BioLabs has grown successful xenograft tumors in the SRG OncoRat® from over 25 cell lines in a variety of cancers. FNA analysis has been done in-house with no negative effects on VCaP prostate cancer cells (Figure 1A), Daudi Burkitt’s Lymphoma cells (Figure 2), and H358 Non-Small Cell Lung Cancer (NSCLC) cells (Figure 3A). With extensive cell models available, Hera can sample the tumor with FNA throughout treatment using your drug or chemotherapy of choice, enabling thorough pre-clinical data in a minimal number of animals. FNA sampling is available at any time point of your choice after dosing – we can help you measure RNA changes 12 or 24 hours after treatment, protein changes via western blot, histological tumor microenvironment changes, or any time-sensitive parameter that your unique study requires. Successful western blots have been completed by clients on FNA samples collected at Hera, with positive results demonstrated for different proteins of interest (Figure 1B).
Samples obtained by FNA retain an extremely high cellular viability even after overnight shipping to your research lab. Independent analysis by a third party CRO has found that up to 96% of cells from the aspirate are viable after shipping (Figure 3B). Retaining cellular viability allows seamless off-site analysis at either your institution or an independent organization, including assays done on living cells. Of course, aspirates can also be flash frozen or fixed before shipment to best prepare the sample for your individual downstream research needs.
Contact Hera today to improve your preclinical xenograft studies with increased data collection through FNA sampling. Our OncoRat® model is specifically designed for multiple preclinical uses, marked by toxicology capabilities, pharmacokinetic studies, and increased tumor volumes that easily support FNA analysis to aid your research study needs.
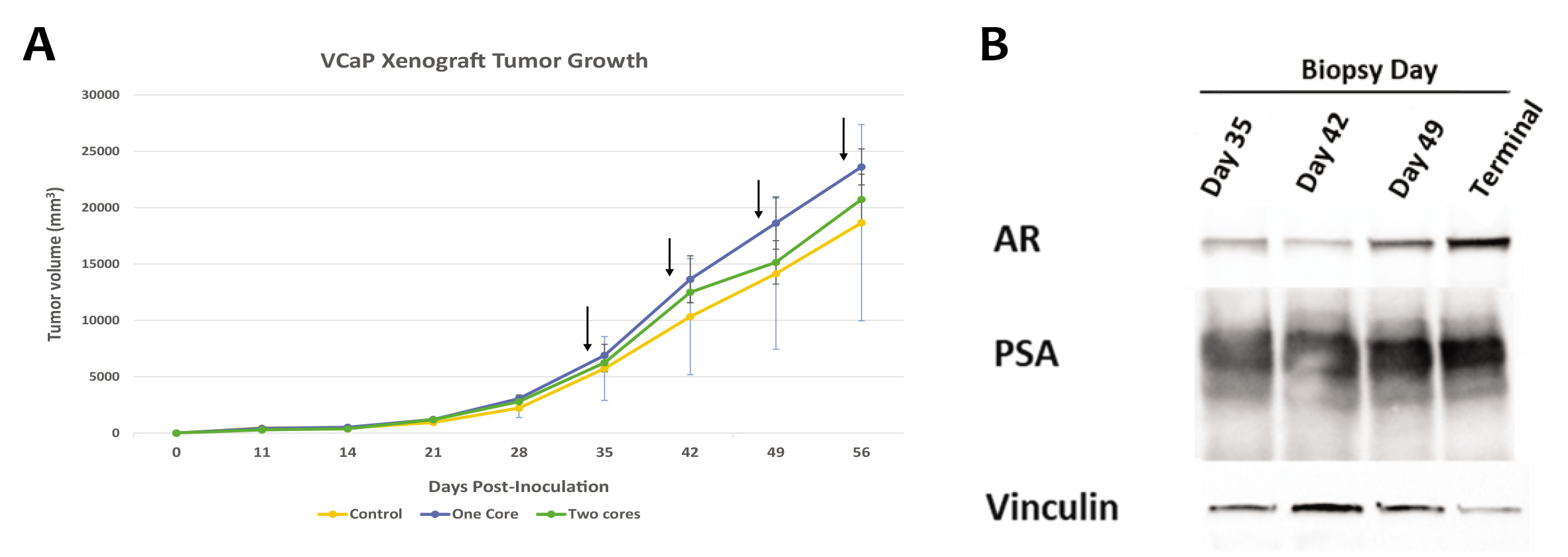
Figure 1: (A) FNA sampling does not significantly affect tumor growth kinetics of VCaP prostate cancer cells injected subcutaneously into the flank of the SRG OncoRat®. Control (no FNA), one core (one FNA), and two cores (two FNA from the same tumor) samples were taken weekly at points indicated with black arrows. Each group is representative of three animals, and error bars indicate SEM. (B) Western blot analysis of FNA were assessed for prostate specific antigen (PSA), androgen receptor (AR), and vinculin expression.
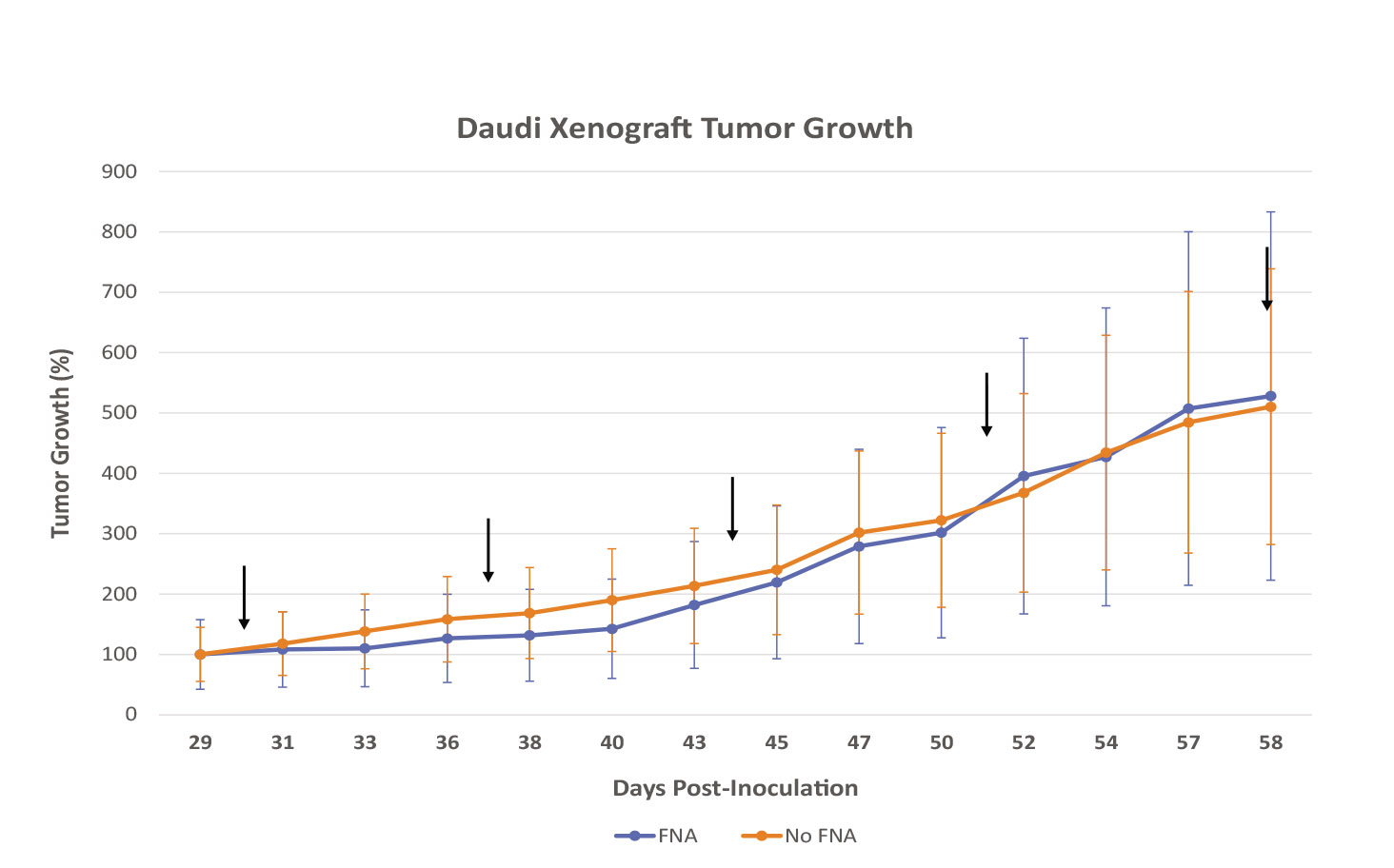
Figure 2: FNA sampling does not have a significant effect on tumor growth kinetics of Daudi Burkitt’s Lymphoma cells injected subcutaneously into the flank of the SRG OncoRat®. FNA group (n=3) underwent weekly biopsies at points indicated with black arrows, non-FNA group (n=5) did not undergo sham procedure. Error bars indicate SEM. No statistical significance between groups as determined by unpaired t-test.
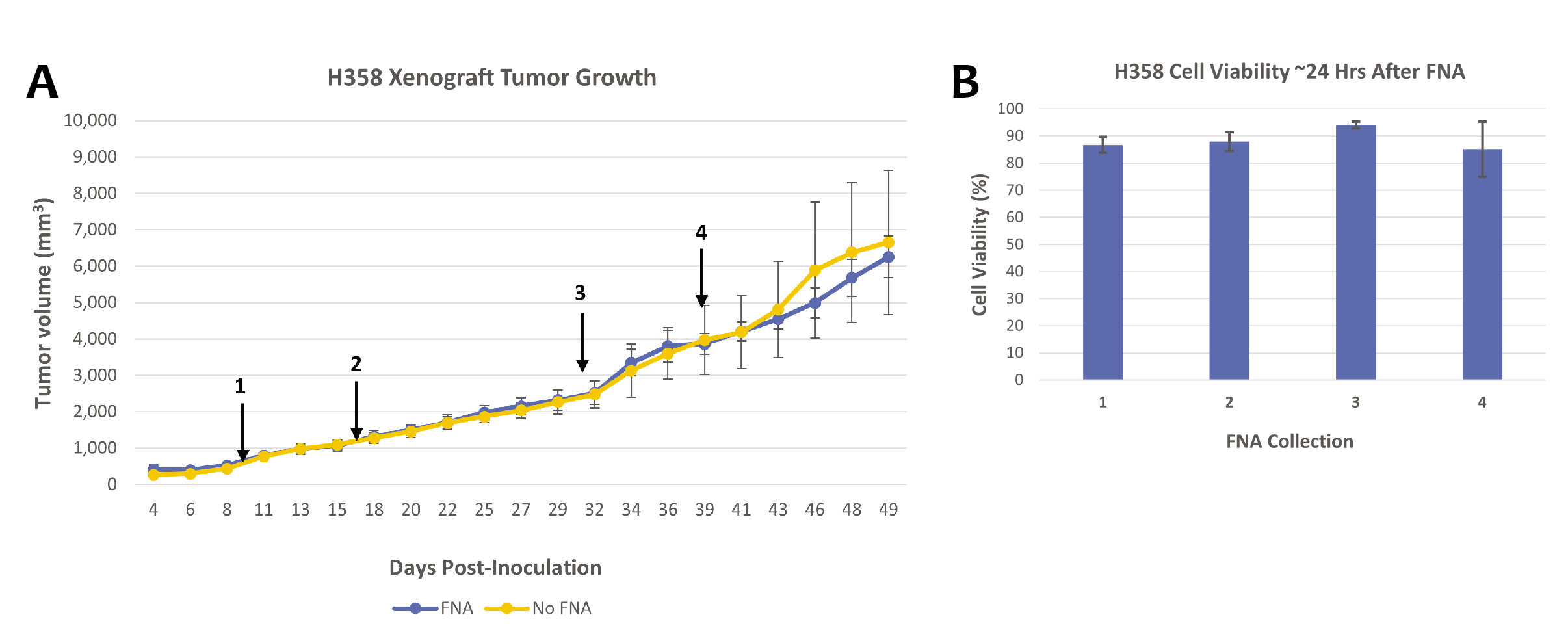
Figure 3: (A) H358 Non-Small Cell Lung Cancer (NSCLC) cells injected subcutaneously into the flank of the SRG OncoRat® have persisting large tumor volumes while undergoing FNA procedures. Tumors were sampled via FNA at points indicated with black arrows. Groups are representative of three animals, and error bars indicate SEM. (B) FNA samples retain very high cellular viability. Samples from (A) were obtained, placed in 1mL RPMI-1640 + 10% FBS and shipped overnight at 4°C for third party analysis. Each collection consists of one FNA sample from three xenograft tumors, error bars indicate SEM.
Contact Us to Learn More
If you would like to explore how the SRG Rat can help accelerate your oncology research, please contact us here.
References
- Noto, Fallon K., et al. “Abstract B067: A Rag2/Il2rg double-knockout rat (SRG OncoRat) enables precision-medicine based cancer studies with cell line-and patient-derived xenografts.” (2019): B067-B067.