One of the most versatile and widely adopted transposon systems, the piggyBac transposase + transposon platform is a TTAA-specific, cut-and-paste gene editing technology. It functions efficiently independent of host cell factors and has a high propensity for integration into highly transcribed units. These features make piggyBac a superior tool for insertional mutagenesis or gene of interest (GOI) expression and precise genome engineering through seamless excision.
- Small to enormous gene cargo integration (200kb+)
- Very efficient
- High expression
- Stable, seamless removal if desired
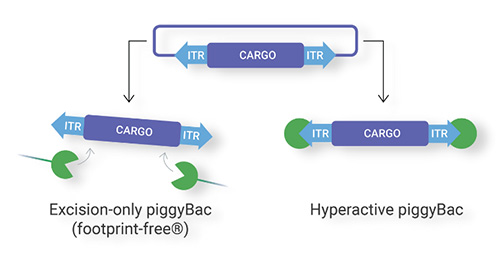
Overview of piggyBac
One study describes the modified versions of piggyBac transposase. They have potentially wide-ranging applications, such as reversible transgenesis and modified targeting of insertions. piggyBac is distinguished by its ability to excise precisely, restoring the donor site to its pre-transposon state. This characteristic is what makes piggyBac useful for reversible transgenesis, a potentially valuable feature when phenotype reversion is desired.¹
Additionally, the piggyBac transposase can insert very large cargo sequences, with capacities of 200kb+ at high frequencies and across a vast array of organisms. In contrast to most other transposon systems, piggyBac has no overproduction inhibition, enabling the use of specialized promotors that are commonly used for gene over-expression. Furthermore, piggyBac is also capable of efficient simultaneous integration of multiple different DNA sequences.²
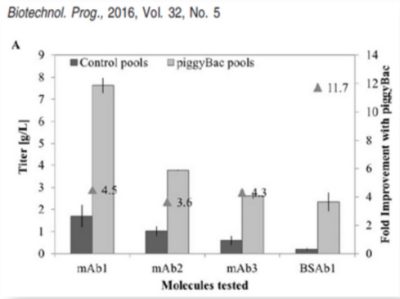
Figure 1:Approximately 4 to 11-fold greater titers compared to conventional gene integration systems for monoclonal antibodies (mAbs) or bispecific antibodies (BSAb).³
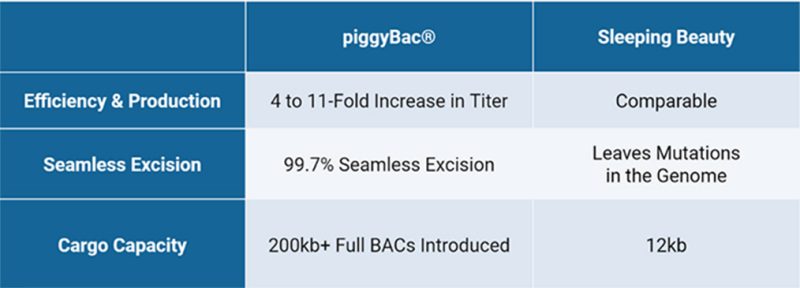
Figure 2: Comparing features of piggyBac to the Sleeping Beauty Transposon
Accelerating Therapeutics Research with piggyBac
Using piggyBac can vastly accelerate gene editing procedures, which is why 400+ peer-reviewed papers have been published as a result of the piggyBac technology. It quickly delivers stable cell lines
¹ Xianghong Li, Erin R. Burnight, Ashley L. Cooney, Nirav Malani, Troy Brady, Jeffry D. Sander, Janice Staber, Sarah J. Wheelan, J. Keith Joung, Paul B. McCray Jr., Frederic D. Bushman, Patrick L. Sinn, and Nancy L. Craig. piggyBac transposase tools for genome engineering. PNAS June 18, 2013 110 (25) E2279-E2287; https://doi.org/10.1073/pnas.1305987110
² Wagner, J., Williams, E., Alper, H. (2018). Developing a piggyBac transposon system and compatible selection markers for insertional mutagenesis and genome engineering in Yarrowia lipolytica. McKetta Department of Chemical Engineering, The University of Texas at Austin. https://doi.org/10.1002/biot.201800022
³ Rajendra et al. (2016) Generation of Stable Chinese Hamster Ovary Pools Yielding Antibody Titers of up to 7.6 g/L Using the piggyBac Transposon System. Biotechnol. Prog., Vol. 32
