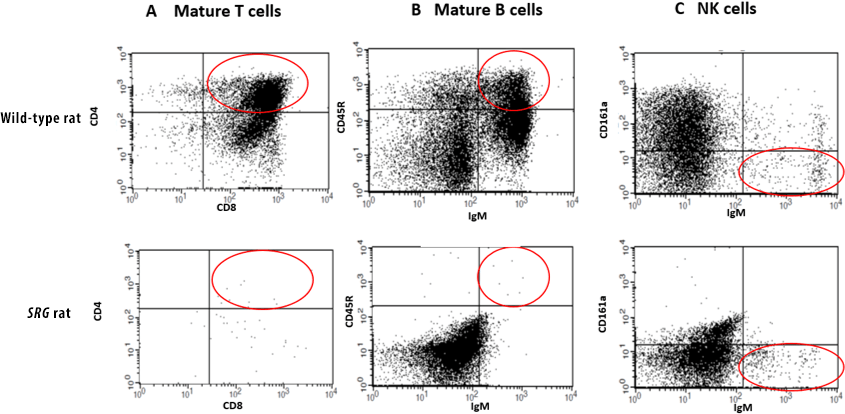
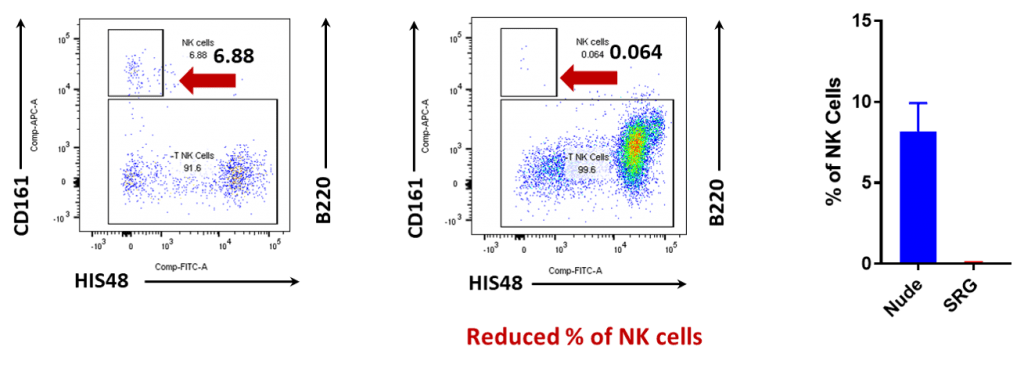
The SRG rat is a SCID model created through knockout mutations in the Rag2 and Il2rgamma genes that result in a loss of mature B, T, and NK cells. the SRG Rat has an immunodeficient phenotype ideal for the engraftment of human tissue.
Taking advantage of the Sprague Dawley background and the larger organism size, SRG users have found our rats uniquely optimal for combining efficacy, pharmacokinetic (PK), biomarker, and toxicology related endpoints.
Get the most out of the SRG Rat model by leveraging our preclinical oncology expertise. In the hands of our seasoned researchers, the rat can deliver robust data, faster.
What’s Unique About The SRG Rat Model?
While some immunodeficient rodent strains such as the Nude rat are partially immunodeficient missing T-cells, this enhanced immunodeficient rat model has a severely impaired immune system lacking B-cells, T-cells, and NK-cells.
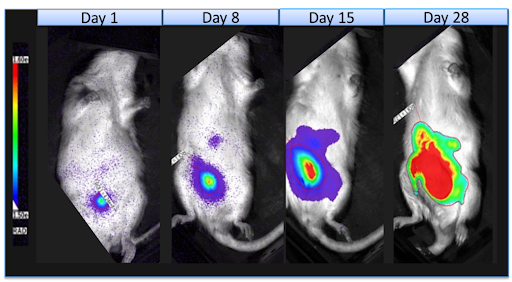
The SRG Rat model demonstrated enhanced utility such as high tumor takes and favorable growth kinetics for both cancer cell-line xenografts and PDX models. The model overcomes the limitations of smaller mouse models enabling larger tumor growth, easier surgeries, and more tissues and serum for analyses, including repeat sampling.
Xenograft & PDX Studies
Hera provides in vivo oncology studies in our SRG rat in wide variety of xenograft and PDX tumor models. Learn more about why researchers prefer our SRG rat over traditional mouse models.
Or request more information about our capabilities and expansive xenograft portfolio. Let our team of expert scientists advise on the selecting the perfect preclinical drug efficacy testing strategy.
Exclusive SRG distribution partnership
with Charles River

Hera has partnered with Charles River for the distribution of the SRG rat to the directly to the global research community. Now all rat orders will be placed with and fulfilled by Charles River.
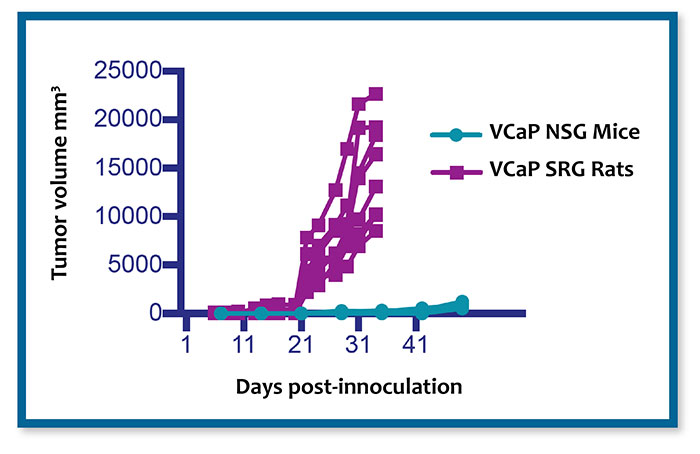
Validated Using Difficult Xenograft and Patient Derived Xenograft (PDX) Models
The SRG Rat has been validated using a wide range of xenografts that consistently demonstrate high tumor take rates with traditionally difficult to engraft cell lines, such as VCaP prostate cancer, H358 non-small cell lung cancer (NSCLC), and prostate, NSCLC, and ovarian PDX models.
Additionally, humane endpoints allow for subcutaneously implanted xenograft and PDX models to reach 10X tumor volumes, up to >20,000 mm3, compared to mouse xenografts allowing for serial tumor sampling or faster PDX establishment.
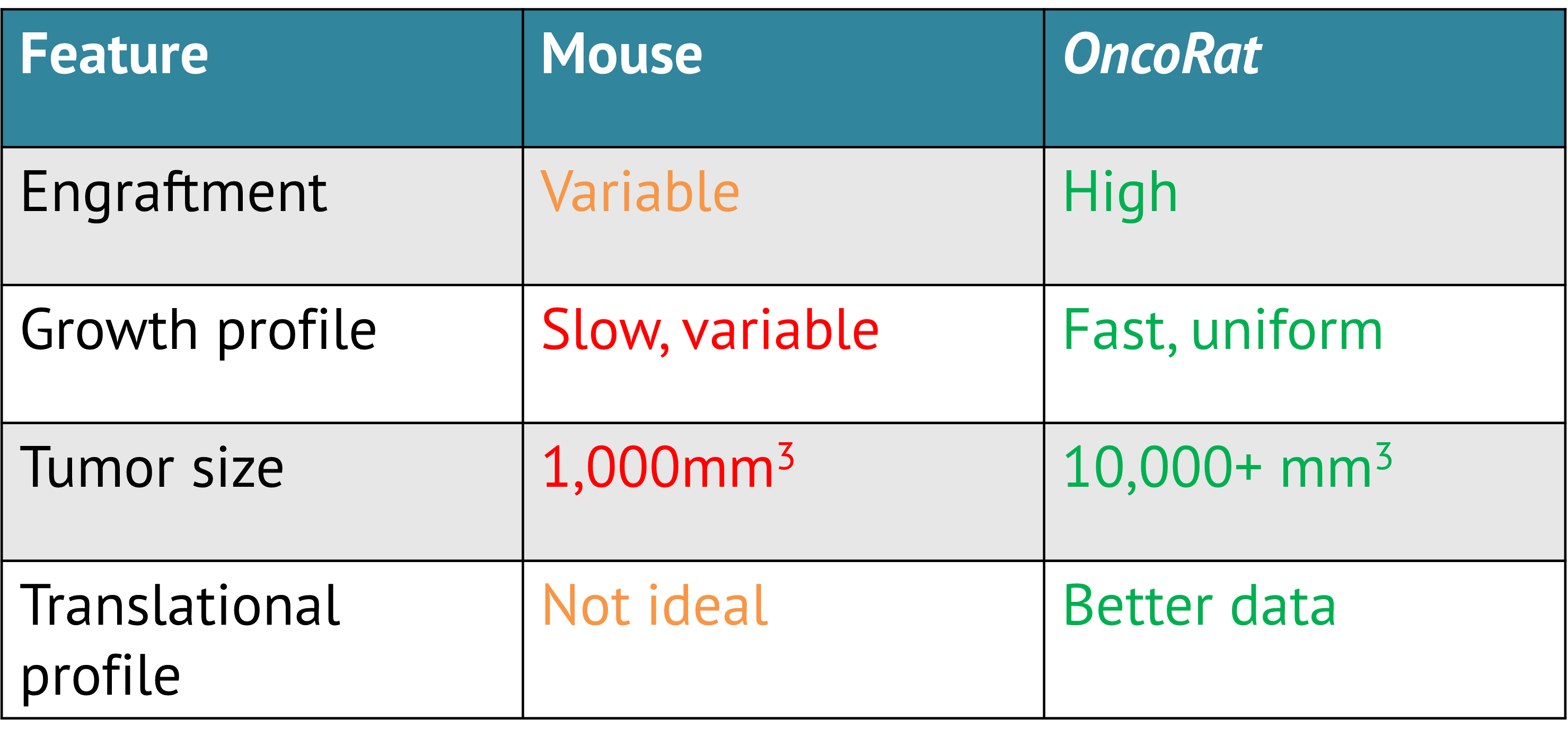
How Is The SRG Rat Better Than SCID Mice?
Through the use of the SRG Rat, researchers can collect more consistent, higher quality data that provides advantages for predicting success in the clinic.
Xenografts in the larger SRG Rat models can display vastly improved engraftment efficiencies, tumor size, growth, and tumor morphology across a number of human cancer cell lines and patient-derived tissues that are either not permissive or extremely inefficient in mice.
Cancer Researchers Find Success With SRG Rat Models
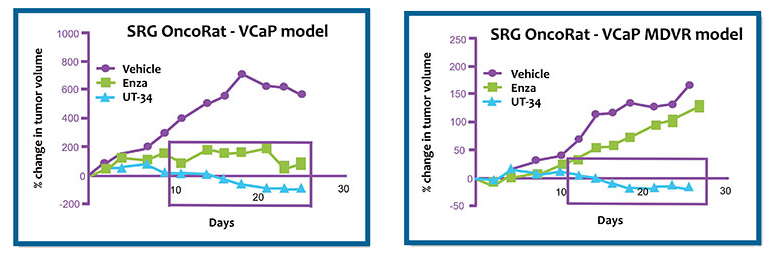
A team of researchers led by Dr. Ramesh Narayanan at the University of Tennessee partnered with Hera BioLabs to evaluate new therapeutics for castration-resistant prostate cancer (CRPC), targeting androgen receptors (ARs).
Given the need for prostate cancer xenografts which model high AR-expression amplification phenotype found in many CRPC patients, the SRG rat has high tumor take rates of prostate cancer xenograft cell lines such as LNCaP & VCaP (>90%) which are prohibitively inefficient in mouse. Hera has expanded our capabilities to include validated castration-resistant and enzalutamide-resistant models.

“The tumor uptake rate was to 80-100%, which is a huge advantage because what we could achieve as a statistical significance with 8-12 mice, can be achieved with 5-6 OncoRats.”
Ramesh Narayanan, Ph.D.
Associate Professor of Medicine & Hematology
University of Tennessee Health Science Center
Creating the SRG Rat
The SRG Platform, is a Sprague-Dawley rat with a double knockout for the Rag2 and Il2rgamma genes (SD-Rag2tm2hera Il2rgtm1hera ). SRG is a severely immunodeficient rat that lacks B-cells, T-cells, and NK-cells. The OncoRat® was the first model built on the SRG Platform and introduced to enable and accelerate xenograft efficacy studies.
Background
Cutting-edge gene-editing technologies Cas-CLOVER™ and piggyBac™ enable Hera to precisely engineer animal models and cell systems. Due to limitations of mouse models for oncology and the improved translatability of rats; Hera set out to create the ultimate rat model for oncology. The SRG Rat Platform was created through targeted-nuclease mediated gene disruption of the rat Rag2 and II2rg genes. It contains an 8 bp deletion in the Rag2 gene causing defective V(D)J recombination preventing T cell and B cell development. SRG also has a 16 bp deletion in the Il2rg gene, which leads to a lack of cytokine signaling, resulting in defective lymphoid development. The combined mutations result in a SCID rat with loss of mature B, T, and NK cells.
Phenotype & Applications
The SRG has a SCID rat phenotype and accepts human tissue as a xenograft model. The SRG Rat (or OncoRat) has been validated with a wide range of xenograft models including patient derived xenografts (PDX) and consistently demonstrates improved tumor take-rates and delivers tumor sizes 10x that of mouse models in half the time. For translational research, the rat produces blood and tissue samples ten times larger than mice and is metabolically closer to humans. Since the rat is the preferred model for pharmacokinetics (PK) and toxicology, OncoRat is ideally suited for efficacy and PK/toxicology studies in the same animal.
The SRG Platform can also be reconstituted with human immune cells such as PBMCs to produce humanized rats, the ImmunoRat™ – is in development for immune-oncology studies and in pharmacology and toxicology with a humanized hepatocyte SRG Model – HepatoRat™. Hera and its collaborators continue to develop additional applications of the SRG Platform. Please contact us if you have questions about applications outside of oncology and immune-oncology.