About Hep B3
Hep B3 is a well-established human hepatocellular carcinoma (HCC) cell line that originated from a pediatric liver tumor in 1979. These cells are cultivated under adherent culture conditions and display an epithelial morphology, resembling the characteristics of hepatocellular carcinoma. Hep3B cells harbor specific genetic mutations in the TP53 and CTNNB1 genes, which play pivotal roles in the development and progression of HCC. The TP53 gene encodes the tumor suppressor protein p53, which is responsible for regulating cell cycle progression and preventing the formation of cancerous cells. The presence of mutations in TP53 in Hep B3 cells suggests an aberration in p53-mediated tumor suppression mechanisms.
Additionally, the CTNNB1 gene encodes β-catenin, a critical component of the Wnt signaling pathway. Dysregulation of this pathway, frequently observed in HCC, can lead to abnormal cell growth and division. The presence of CTNNB1 mutations in Hep3B cells implicates the involvement of Wnt signaling dysfunction in HCC development. Another noteworthy characteristic of Hep B3 cells is their elevated expression of alpha-fetoprotein (AFP), a tumor marker commonly associated with HCC. AFP serves as a clinically valuable diagnostic indicator for HCC and its overexpression in Hep3B cells further validates their relevance as an HCC model system.
Regarding drug sensitivity, Hep3B cells have demonstrable sensitivity to ulixertinib, motesanib, and AGI-5198. These findings indicate that these specific compounds may hold potential as therapeutic agents against HCC.
Hep B3 Tumor Kinetics in the SRG™ Rat
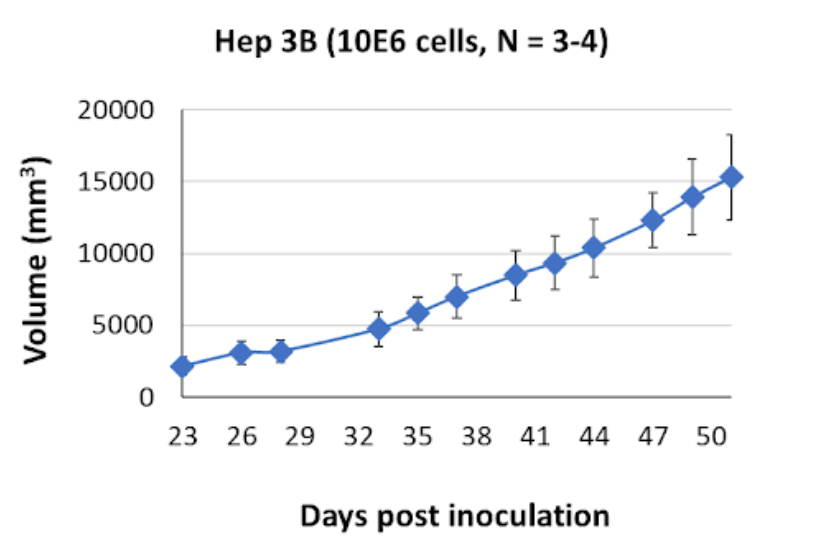
In vivo studies utilizing xenograft SRG rat models have shown that Hep B3 cells form solid, well-defined tumors with a firm consistency. These tumors exhibit prurient growth patterns, meaning they extend aggressively and can invade nearby tissues. Additionally, immune infiltrates can accumulate within Hep B3 tumors, suggesting the involvement of immune responses in the tumor microenvironment.
Products & Services
Xenograft Efficacy Studies
Includes collection of blood, tissues & tumor for ADME, PK/PD and analysis.
(Bi)weekly Tumor Sampling
Via fine needle aspiration (FNA). For longitudinal evaluation of drug exposure, histology and gene expression.
OncoRats
Cutting edge models optimized for engraftment.
Get help with your research by scheduling a call with Hera.
References (MLA):
- Biterge Süt, Burcu. “Computational Analysis of TP53 vs. CTNNB1 Mutations in Hepatocellular Carcinoma Suggests Distinct Cancer Subtypes with Differential Gene Expression Profiles and Chromatin States.” Computational Biology and Chemistry, 16 Oct. 2020, www.sciencedirect.com/science/article/abs/pii/S1476927120303170?via%3Dihub.
- Fong, Jason T., et al. “Alternative Signaling Pathways as Potential Therapeutic Targets for Overcoming EGFR and C-MET Inhibitor Resistance in Non-Small Cell Lung Cancer.” PLOS ONE, Nov. 2013, journals.plos.org/plosone/article?id=10.1371%2Fjournal.pone.0078398.
- Kashyap, Randeep, et al. “Clinical Significance of Elevated α-Fetoprotein in Adults and Children – Digestive Diseases and Sciences.” SpringerLink, Aug. 2001, link.springer.com/article/10.1023/A:1010605621406.
