We Are Hera BioLabs
Seasoned Researchers Generating Transformative
Data and Products for R&D
At Hera, we leverage a 15+ year history of gene engineering advancements to create better models and be your preclinical research partner of choice. Our proprietary family of platforms includes:
Combined with these proprietary platforms, our experienced team, modern facilities, and portfolio of sophisticated research tools and services deliver robust, high-quality data to accelerate drug discovery and development.
Our Work Supports Advancements In:
Compliment Your Research
with Our Expertise
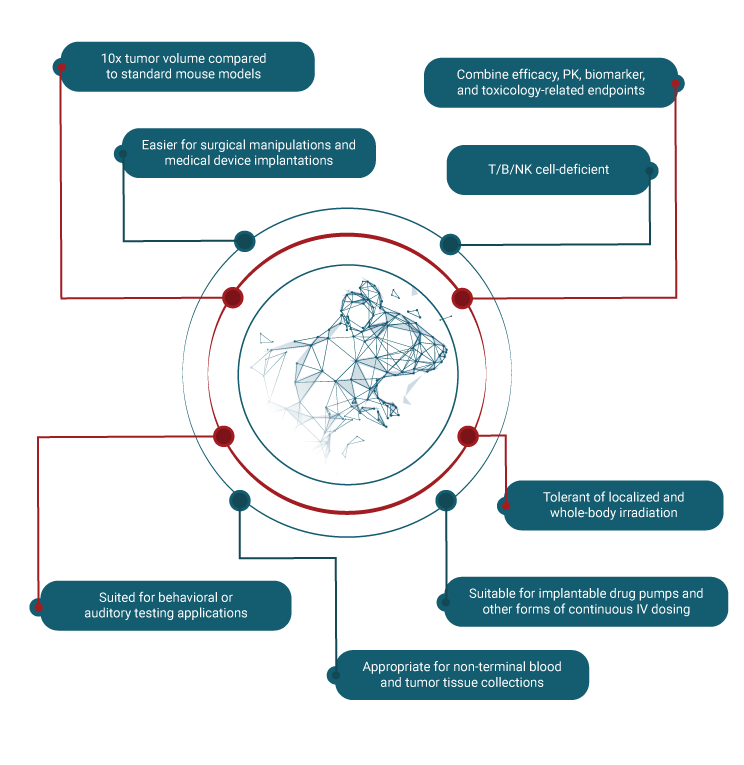
Hera’s SRG Rat®
A Smarter Choice for Oncology Insights
To meet the industry’s need for a larger rodent model with a high probability of attaining successful cell line-derived xenografts and PDXs of sufficient size for serial biopsies, Hera developed and validated a platform in vivo model called the SRG Rat®. A SCID rat of the Sprague Dawley background engineered to house a double knockout of the Rag2 and Il2rγ genes, the SRG Rat® has proven applications in oncology, infectious diseases, and other areas of pharmacology/toxicology.
To further accelerate your R&D, our team provides in vivo services at Hera BioLabs and rapid delivery of the SRG Rat through the global network of Charles River.
Researcher Spotlight
Prostate Cancer Research with the SRG Rat®
“The tumor uptake rate was 80-100%, which is a huge advantage because what we could achieve as statistical significance with 8-12 mice, can be achieved with 5-6 SRG Rats®.”
Ramesh Narayanan, Ph.D. Associate Professor of Medicine & Hematology at the University of Tennessee Health Science Center
Advance Your Gene Editing
Make Progress with Our Novel Technologies
Gene editing expertise and proprietary technology are core to Hera’s history and the secret to our advanced gene-editing toolbox. Our platforms’ portfolio of successes includes building 100+ custom-engineered cell lines- from knockouts and knock-ins to stable cell lines and reliably accelerating R&D activities.
Hera’s Lexington Facility
AAALAC Accredited. Independent. US-Based.
Strategically located, our headquarters and facility in Lexington, Kentucky, USA, house the latest in gene editing technology and animal care. Our on-site barrier-level vivarium was designed to house immunodeficient models and features the Innovive Innorack® and Innocage® dual HEPA-filtered, disposable IVC rodent caging system.
Our Commitment
To The Animals in Our Care
We are grateful to the animals who have helped us attain critical preclinical data to move projects into clinical development for the improvement of patient lives. We are devoted to their ethical care and honor their service to science.
Our AAALAC Accredited Animal Welfare Program and our commitment to fostering a culture of care for the animals we work with are deeply rooted in the Replacement, Reduction, and Refinement ethical framework of modern research.
If you would like to learn more, please visit our Facilities/Vivarium page.
“The Council commends [Hera for] maintaining an exemplary program of laboratory animal care and use. Especially the [team’s] strong administrative commitment to the program and to attaining accreditation, …the investments in infrastructure, equipment, and personnel, [and their] excellent documentation, and standard operating procedures…”

Our Latest
Insights From Hera’s Team
Learn from our experts with thought leadership content about Hera’s latest R&D efforts and CRO industry trends.
Genetically Immortalized Cells: Unlocking the Potential for Scalable Cultured Meat Production
Genetically Immortalized Cells: Unlocking the Potential for Scalable Cultured [...]
How the piggyBac® Transposon Sheds Light on Breast Cancer Drug Resistance
With over 2.2 million annual cases, breast cancer is the [...]
The MCF-7 Breast Cancer Model: A Definitive Guide
In this guide, we provide a description of the [...]