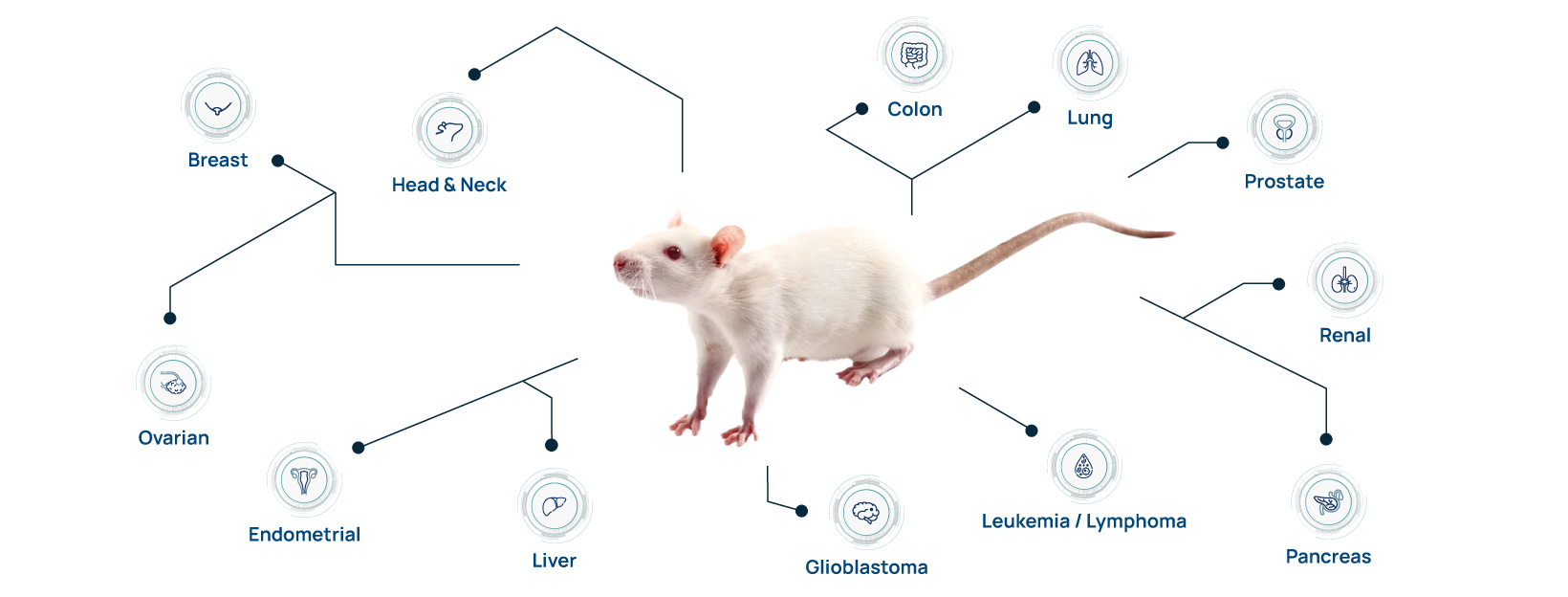
Find the Right Xenograft Model
Hera provides in vitro and in vivo oncology studies to leaders in industry and academia. We have optimized a wide variety of xenograft, PDX, and syngeneic tumor models in both mice and our unique SRG rat OncoRat.
Request more information about our capabilities and expansive xenograft portfolio.
Identifying the right preclinical efficacy model involves a lot of choices: subcutaneous or orthotopic, mouse or rat xenograft host, cell line xenografts or patient-derived xenografts (PDX), and humanized immune system vs syngeneic. Let our team of expert scientists advise on the selecting the perfect preclinical drug efficacy testing strategy.
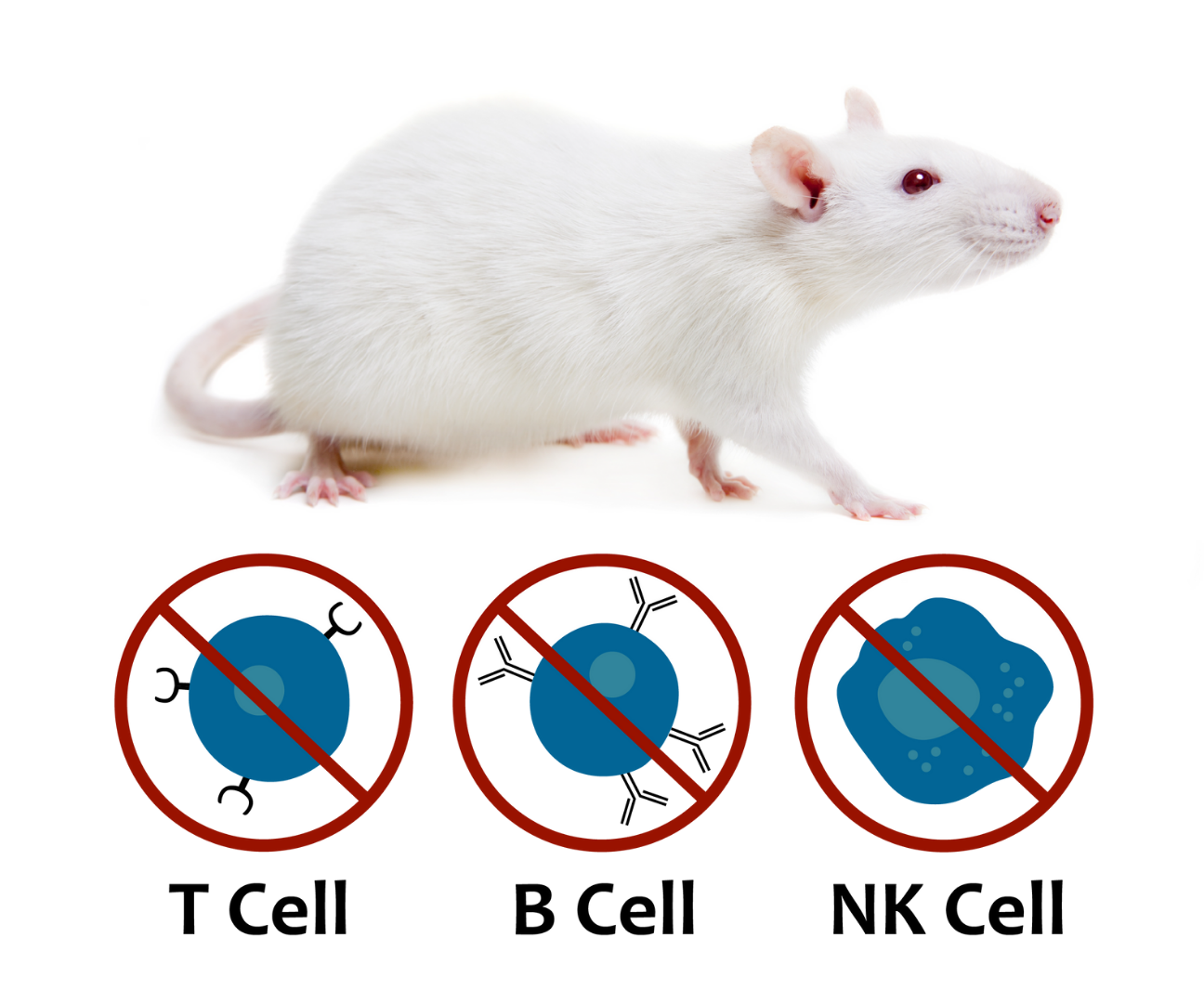
The SRG Rat: Building a Better Trap for Cancer
The SRG rat is an ideal host for human xenografts. It is a highly immunodeficient (SCID) rat on the Sprague-Dawley background similar to NSG mice, demonstrating enhanced immunodeficiency, lacking B-cells, T-cells, and NK-cells.
Hera offers xenograft studies in our SRG rat which provides several advantages over traditional mouse models. It demonstrates high tumor take-rates for difficult cancer types (both PDXs and cell line xenografts), it allows for easier surgical manipulation, increased blood/tissue volume and the ability to combine drug efficacy with metabolism/toxicology endpoints in the relevant Sprague Dawley background. Learn how using the SRG is making oncology studies more reliable, efficient & robust.
Mouse Oncology & Immuno-Oncology Services
Partnering with us not only gives you access to unique SRG rat models but broadens your options with an experienced provider of mouse in vivo services on many backgrounds including NSG, NCG, NOG, and the nude mouse.
Our goal is to deliver the best data possible for our clients. We routinely use both rat and mouse for in vivo oncology and provide advanced immuno-oncology services in mice. Our immune-oncology platform uses mice engrafted with CD34+ hematopoietic stem/progenitor cells (HSPCs), a validated platform providing bone marrow engraftment and development of multi-lineage human immune cells.
We offer standardized humanized mouse models or your specific donor, whether HLA matching or engineered cells for your preclinical studies.
Applications & Case Studies
Find related and up-to-date information and literature for our In Vivo Oncology service
including blogs, posters, presentations, webinars, and white papers.
-
 Advantages of Utilizing the SRG rat for Investigating Tumor Metastasis
-
Read More →
Advantages of Utilizing the SRG rat for Investigating Tumor Metastasis
-
Read More →
-
 Validation Of The SRG Rat For Human Tumor Studies Published In PLOS ONE
-
We Are Excited To Announce That A Validation Study Using […] Read More →
Validation Of The SRG Rat For Human Tumor Studies Published In PLOS ONE
-
We Are Excited To Announce That A Validation Study Using […] Read More →
-
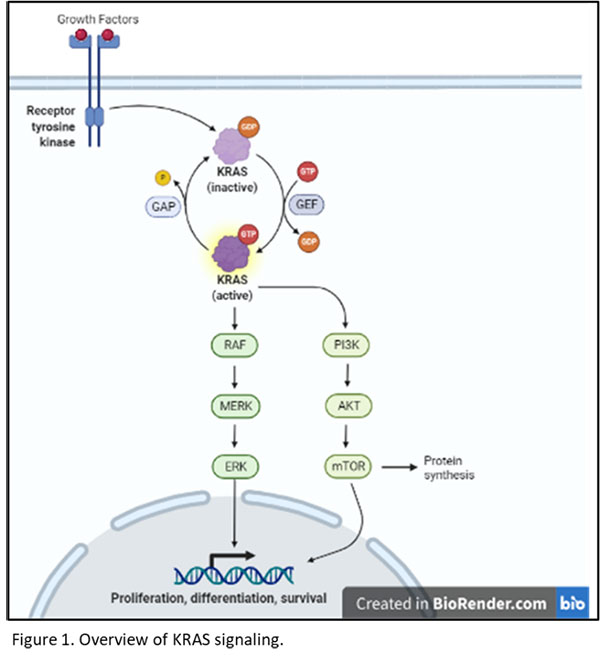 In Vivo Targeting Of Mutant KRAS Using Human Cancer Xenografts In The Immunodeficient SRG OncoRat®
-
Targeting KRAS In Oncology Research? KRAS has become an important […] Read More →
In Vivo Targeting Of Mutant KRAS Using Human Cancer Xenografts In The Immunodeficient SRG OncoRat®
-
Targeting KRAS In Oncology Research? KRAS has become an important […] Read More →
Accredited Facility Leveraged By Our Talented Team
Located in Lexington, KY, our state-of-the art facility include both our molecular biology laboratory and vivarium from which we provide all our contract research services.
Our barrier status animal facility includes an AAALAC accredited animal welfare program with the Association for Assessment and Accreditation of Laboratory Animal Care, and Hera maintains an OLAW/PHS Assurance (NIH Public Health Service).
To further facilitate comprehensive scientific teams, we hold memberships and training programs with the NIH (National Institute of Health), the University of Kentucky’s Division of Laboratory Animal Resources (DLAR), and the American Association of Laboratory Animal Science (AALAS).


Xenograft vs PDX
Researchers are often asking our scientific team about how to choose between using a cell line xenograft and a patient derived xenograft (PDX). This article aims to summarize the pros and cons of each approach and allow you to identifying which model type is the best fit for your drug development needs.
The etymology of xenograft begins with Xeno- which is a Latin derived prefix meaning “foreign.” Xenografts can be defined as the transplantation of foreign (i.e. different species) tissue into a recipient host. For oncology studies, this is commonly either an immortalized, homogenous cancer cell line comprising of one cell type or tissue that is collected directly from the cancer patient that includes a heterogenous cell population and transplanted into an animal host. The xenograft host for this type of research is typically an immunodeficient or SCID rodent model, such as the NSG mouse or the SRG rat. When the engraft tissue is sourced directly from the patient and isn’t passaged in cell culture, it is referred to as a patient-derived xenograft (PDX).
Why use a cell line xenograft?
Most preclinical oncology drug efficacy studies are conducted in cell line xenografts because they are more consistent, characterized, and cost effective. Commercially available cell lines (from a repository such as ATCC) are highly standardized allowing for comparisons to data and peer-reviewed publications across the entire field including genotyping and gene expression data. In vitro cell line screens can identify highly susceptible cancer types or particular lines which can be then used implanted as xenografts.
Xenograft cell lines when implanted in rats or mice and generally have high tumor take-rates and growth kinetics across with less study-to-study and animal-to-animal variability. The cell lines characteristics are static and preserved, can be cultured whenever needed, and maintained in repositories providing easy access. This is ideal when compared to a PDX, which requires time and resources to cyrorecover the PDX model or continuously passage in-vivo. PDXs can have more variable tumor uptake and growth kinetics, and their characteristics can drift after multiple passages.
Why use a PDX?
Researchers believe that using a patient derived xenograft model may provide more translatable data by preserving the original tumor complexity and heterogeneity. It has been shown in numerous publications that the original patient tumor heterogeneity is well preserved in initial passages, and PDX treatment data can be more predictive of human drug responses- opening the door to patient-avatar or precision medicine approaches. PDXs often capture rare tumor types, provide interesting mutation profiles, and can answer questions about specific patient populations (to assist in clinical trial enrollment criteria for example).
It has been shown in several publications, including Sato et. al, that at each passage (from animal to animal) the tissue heterogeneity decreases and the number of cell types present, gene expression profiles, and mutation status starts to shift from the original patient sample.
Hera is tackling this problem in two ways:
- Using our SRG rat to establish PDXs faster and larger allowing more utility at earlier passages and
- Using the rat as xenograft host to address the issue of translatability to the clinic.
Additional disadvantages of PDXs include higher costs, limited access, and longer timelines. Because PDXs are maintained as tumors in a xenograft host and continuously passaged they are costly to maintain or they need to under a cryo-recovery step (3 months). Additionally, each time a PDX is passaged, it needs to be re-characterized to ensure the tumor retains the same mutation profile and histological characteristics.
At Hera, we view PDXs as an extremely important tool to address very specific research questions, or examine patient populations, model rare cancer types, etc, but because of the added cost and timeline, we recommend first demonstrating successful in vivo drug efficacy with a standard cell line xenograft model.
Increasing Translatability
To increase the translatability of drug candidates, Hera recommends using our SRG rat as the xenograft hosts for your research studies. For both cell line xenografts and PDXs our SRG rat has been shown to have a more human-like tumor microenvironment including important difference compared to the mouse in fibrotic tissue and angiogenesis (ask us about it). The rat also has a more human-like drug metabolism leading to translational tumor exposures and prevalence of potential systemic toxicity issues. The SRG rat is highly versatile in many areas of research including drug efficacy, toxicology, and metabolism.
Want to be the first to know about groundbreaking research technology updates?
Subscribe to our mailing list to stay in the know.
Why Rats as a Xenograft Model?
A xenograft model is a surgical graft of tissue from one species to another and commonly utilizes an immunodeficient host animal to prevent transplant rejection. Mice and rats with varying degrees of immune system deficiencies can be used for xenografts, but the preferred model is fully immunocompromised, lacking B-cells, T-cells and natural killer (NK) cells. Xenografts in these rats and mice of patient derived cells or primary tumor tissues (PDX, PDTT, or PDTX) provide a better model that overcomes the limitations of traditional in-vivo models and more sufficiently represent human cancer characteristics especially with regard to metastasis and drug sensitivity. Patients derived xenografts are being used to provide results with better translational potential that have eluded researchers using traditional rodent models.
Rat models that are B & T-cell deficient (Rag2 KO) or fully immunocompromised (Rag2/Il2rg double KO, lack of B, T and NK-cells) can be used for xenografts, tumor immunology and PDX studies. Rat models provide several significant advantages for xenograft studies over their complimentary mouse xenografts.
Larger Size:
The larger size of the animals allows for easier handling and more precise surgical manipulations. For example, intracranial xenografts and other orthotopic tissue injections are easier to perform.
More Tissue:
Due to the size of the rats compared to mice, xenograft tumors can grow larger, especially in xenografts into the brain, prostate, and other tissues, and this provides more tissue to analyze.
Easier Tumor Imagining/ Visualization:
Tumor metastasis and angiogenesis are often the primary focus of cancer research and therefore non-invasive in-vivo imagining is becoming an increasingly important tool to understand these aspects of the cancer progression. Rats have been shown to be a better model for in-vivo imagining of metastatic tumors from xenografts which are easier to detect due to the larger size[4].
More Utility:
The rats can have multiple blood draws, compared to mice, which allow for drug studies and provides more utility. Additionally, since rats are the preferred model for toxicology, this allows for both efficacy studies and toxicity to be evaluated in the same species.
Sources:
- Yang, et al. A novel GFP nude rat model to investigate tumor-stroma interactions. Cancer Cell International. 2014 December. 14:54
- Asamoto, et al.Prostate Carcinomas Developing in Transgenic Rats with SV40 T Antigen Expression under Probasin Promoter Control Are Strictly Androgen Dependent. Cancer Research. 2001 June 15. 61; 4693
- Iannaccone, P. M., & Jacob, H. J. (2009). Rats! Disease Models & Mechanisms, 2(5-6), 206–210.
Nofiele, et al. Establishment of a Lung Metastatic Breast Tumor Xenograft Model in Nude Rats.. PLoS ONE. 2014 May 16. 9(5), e97950.

