Immunodeficient mice engrafted with CD34+ hematopoietic stem/progenitor cells (HSPCs) are an attractive humanized mouse model for immuno-oncology efficacy studies, as they are a validated platform that provides bone marrow engraftment with development of multi-lineage human immune cells in the peripheral blood.
For many studies, such as those with engineered cells (e.g. HSPCs and CAR-T), it may be preferential that the study sponsor dictates the donor for CD34+ humanized mice. Hera has demonstrated through effective recipient conditioning, transplantation and flow cytometric (FC) analysis efficient engraftment of engineered CD34+ HSPCs as indicated by high levels of human CD45+ chimerism in the blood and bone marrow. We offer custom donor as well as standardized donor humanized mouse studies for preclinical immuno-oncology.
Immuno-Oncology Services
Our team of experienced scientists provide immuno-oncology services utilizing humanized immune system mouse models with CD34+ cells. Humanized mouse models are designed to closely mimic the human immune system, enabling us to evaluate the efficacy and safety of novel immuno-oncology therapies in a preclinical setting.
- Immune cell profiling and characterization
- In vivo tumor growth and tumor infiltration
- Efficacy and toxicity studies of immuno-oncology therapies
- Mechanistic studies of immune response to cancer
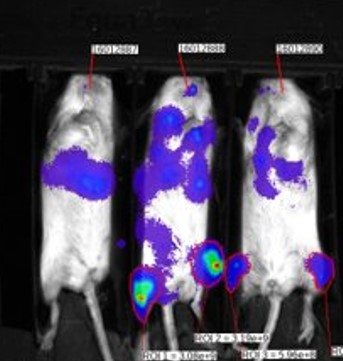
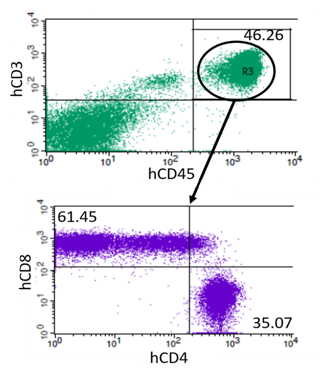
Humanized Immune System in SRG Rats
SRG rats are amenable to engraftment of human immune cells. Having a humanized immune system SRG rats would allow for serial blood sampling, more tumor tissue for studying infiltrating cell populations, and a more translation metabolism. Hera is working to optimize various methods of SRG rat humanization including PBMCs, CD34+ hematopoietic stem cells, and the BLT humanization approach.
Advantages for Immuno-Oncology Research
Mice with a humanized immune system offers several advantages over immunodeficient preclinical models, including:
- Replication of human immune responses and tumor immune cell infiltration.
- Ability to evaluate drug efficacy and toxicity in the context of a human immune system.
- More reliable prediction of clinical outcomes in humans.
At Hera, we are committed to advancing Immuno-Oncology research and helping our clients achieve their goals. Contact us today to learn more about our services and how we can help accelerate your research.

Humanized Immune System in Mice
FC analysis of peripheral blood for human CD45+ at 8 weeks.
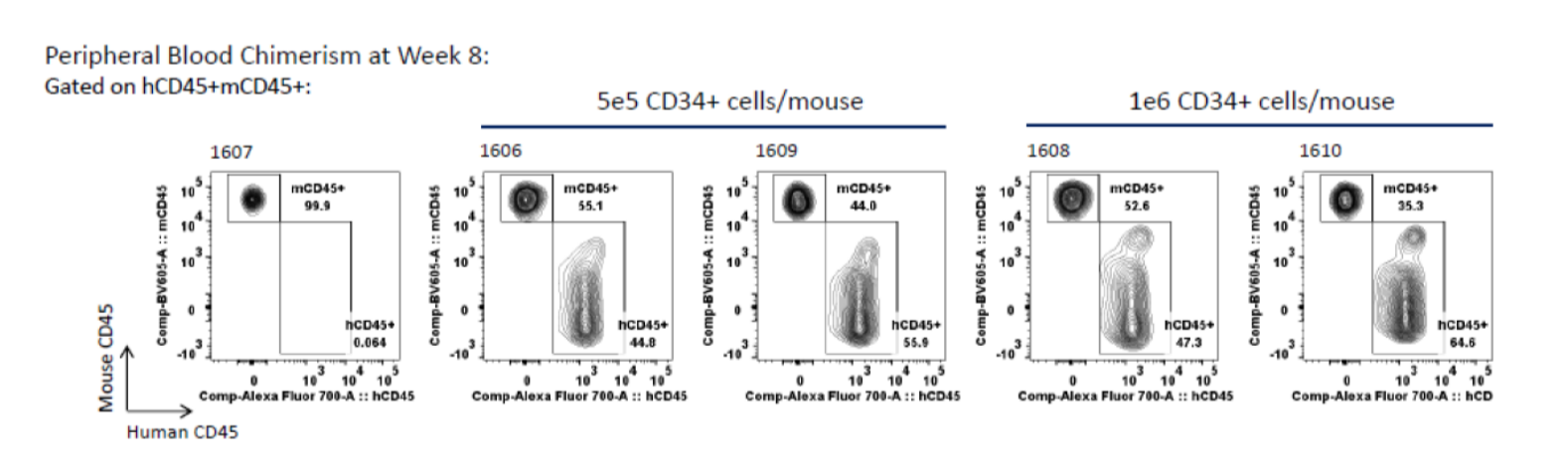
Blood collected at 8 weeks post-transplant of G-CSF mobilized peripheral blood CD34+ cells was subjected to FC analysis for human and mouse CD45. Left panel: group 1 (vehicle control); center two panels: group 2; right two panels: group 3.
FC analysis of bone marrow for human CD45+ at 8 weeks.
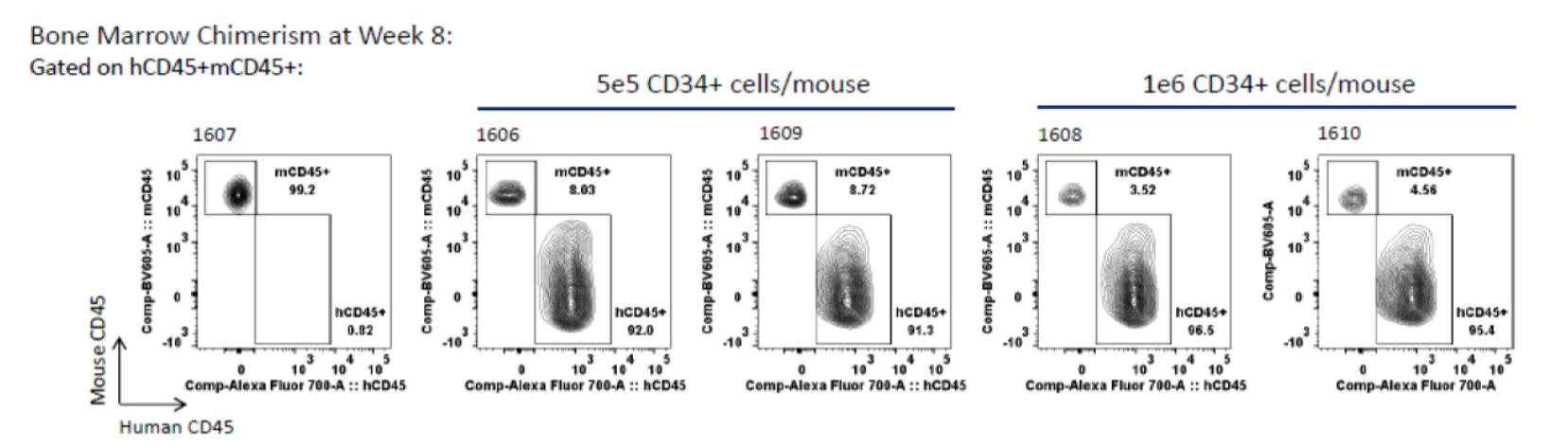
Bone marrow collected at 8 weeks post-transplant of G-CSF mobilized peripheral blood CD34+ cells was subjected to FC analysis for human and mouse CD45. Left panel: group 1 (vehicle control); center two panels: group 2; right two panels: group 3.
