 Orthotopic vs. Subcutaneous Xenograft Models of Human Cancer
Orthotopic vs. Subcutaneous Xenograft Models of Human Cancer
Subcutaneously implanted xenografts or patient-derived xenografts (PDXs) are a commonly used tool in the study of cancer. Orthotopic xenografts are defined as the implantation of cancer cells into the same organ or tissue from which the cancer originated in the human, while subcutaneous xenografts are the implantation of cancer cells under the skin of an immunodeficient mouse or SRG rat. Oncology researchers have differing opinions on which type of model to choose between when comparing orthotopic vs xenografts that are implanted subcutaneously (subq).
Why researchers choose to use a subcutaneous xenografts model:
- Ease-of-use: Subcutaneous xenografts are easier to implant, and monitor compared to orthotopic xenografts, which may require specialized surgical techniques. Subq implantation is an easy to learn technique and is not considered an invasive surgery. Once tumor growth is observed, tumor volumes are easily measured by hand with a caliper enabling efficient tracking of tumor growth.
- Readily available models: Since tumor measurements can be taken by calipers, the xenograft cell lines don’t need to be modified to express fluorescent or bioluminescent genes for assessment of tumor growth and there is a large number of already established models with historic data or publications for comparison when using a subcutaneous model.
- Cost considerations: Because orthotopic xenografts are more technically challenging to perform and require in vivo imaging to monitor tumor growth, researchers choose to not pursue the more resource intensive approach when preparing a cohort of animals for drug efficacy studies.
The advantages of choosing an orthotopic xenograft or PDX instead of using a subcutaneous model:
- The tumor microenvironment of the xenograft or PDX implanted into the organ or tissue of origin may play a role in tumor growth and response to therapy. Orthotopic xenografts are thought to better mimic the native tumor microenvironment, including stromal cell components.
- Tumor growth rate: The rate of tumor growth may differ between orthotopic and subcutaneous xenografts due to differences in the tumor microenvironment and local nutrient supply. This may be particularly important when establishing PDXs which don’t have a high success rate implanted in the subcutaneous space.
- Tumor metastasis: Orthotopic xenografts may more accurately replicate the metastatic behavior of human tumors compared to subcutaneous xenografts.For example, orthotopic xenograft models more closely mimic the metastases observed in human prostate cancer patients, according to a 2016 study published in the Journal of Cellular Biochemistry by Zhang, et al.The results of this study clearly show “very different tumor behavior at the orthotopic and subcutaneous sites of human prostate cancer PC-3 in athymic nude mice,” according to Zhang, et al. “By day-2 after tumor implantation, the orthotopic tumor is already highly vascularized and the cancer cells have begun to migrate out of the tumor. In contrast, the subcutaneous tumor only begins to be vascularized by day-3 and cells to not migrate from the tumor.” Additionally, angiogenesis is much more extensive in the orthotopic tumor compared to the subcutaneous tumor over a two week period.Specifically, the orthotopic PC-3-GFP tumor is observed to grow very rapidly and presents distinct metastases in the lymph nodes by day-3 and evidence of metastases in the abdominal cavity by day-7. Compare this to the PDX model which, after a full 14 days, showed no evidence of invasion or metastasis associated with the subcutaneous tumor, even after the lymph nodes and abdominal cavities of the mouse was extensively explored.Using orthotopically-implanted PC-3-GFP cells, Zhang et al were also able to observe metastatic cells that migrated from the primary tumor to various organ systems, thereby demonstrating that “PC-3 has multiple metastatic routes similar to hormone-independent advanced-stage prostate cancer in the clinic.” As researchers continue to better understand the process by which metastases occur in PC-3 and other tumor types using orthotopic xenografts, the possibility for translational success in improved.
Overall, the choice between orthotopic and subcutaneous xenografts depends on the research question and the specific characteristics of the cancer model being studied. Both methods have advantages and disadvantages, and researchers should carefully consider the relevant factors before making a decision.
The SRG Rat for Orthotopic vs. Subcutaneous Xenografts
Whether you are considering subcutaneous or orthotopic approach to conducting your xenograft or PDX study, the SRG rat can provide certain advantages to both approaches. First, the larger size of the SRG rat makes orthotopic xenograft implantations easier and less prone to error. For example, sub renal/kidney capsule implants or colorectal both considered difficult surgeries, but with a larger rodent host there is more tolerance for successful implantation into the precise target tissue. Secondly, the SRG rat shows a more human-like tumor microenvironment, even in the subcutaneous space, providing a more translational model for drug efficacy studies, and better tumor engraftment and growth kinetics regardless of site of tumor implantation.
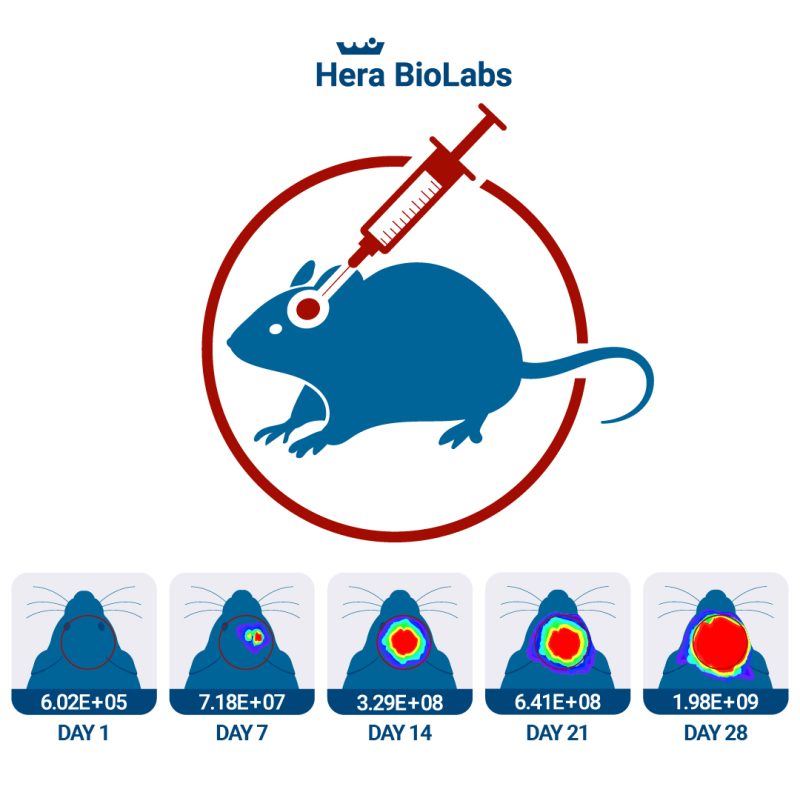
The SRG rat is particularly applicable to the study of glioblastoma or other brain cancers. With these cancer types orthotopic xenografts are essential because having a intact blood-brain barrier is requirement to fully understand drug efficacy. Surgical implantations can be very precise into various rat brain locations (including brain stem) with stereotactic injection equipment and the SRG rat tolerates a high tumor burden allowing for a longer window of study compared to mouse models.
You can learn more about how researchers are using the SRG rat HERE.
