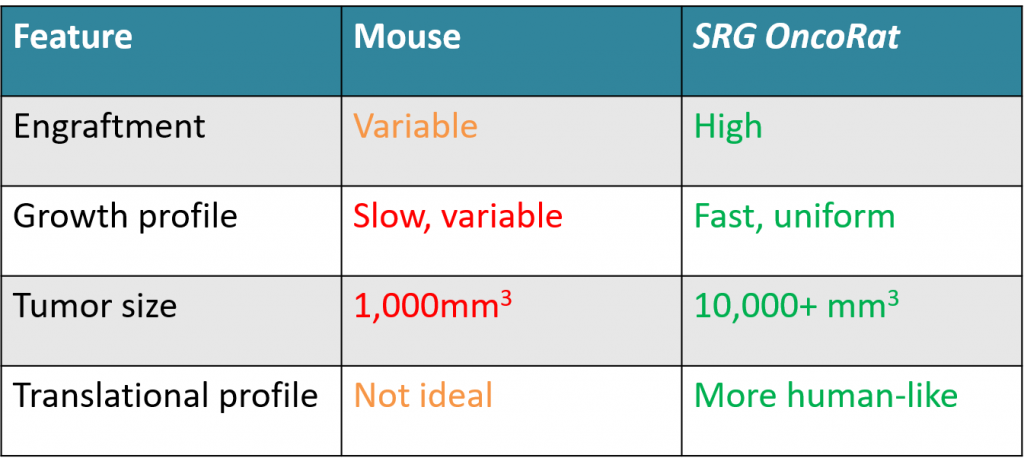
SRG OncoRat is a Sprague-Dawley rat with knockouts for Rag2 and Il2r gamma genes resulting in lack of B-, T-, & NK-cells, a SCID rat. Tumor take rates & growth have demonstrated advantages over SCID and NSG mice, consistently increasing study success while reducing animal numbers (i.e. VCaP prostate, H358 NSCLC & PDX models). Tumors grow ~10x larger enabling PDX establishment in a single passage, shortening timelines and providing more translational studies. Below are some examples of how SRG OncoRat can be used to complement, confirm or in some cases replace your SCID mice or NSG mouse xenograft data.
Improved engraftment enables oncology research
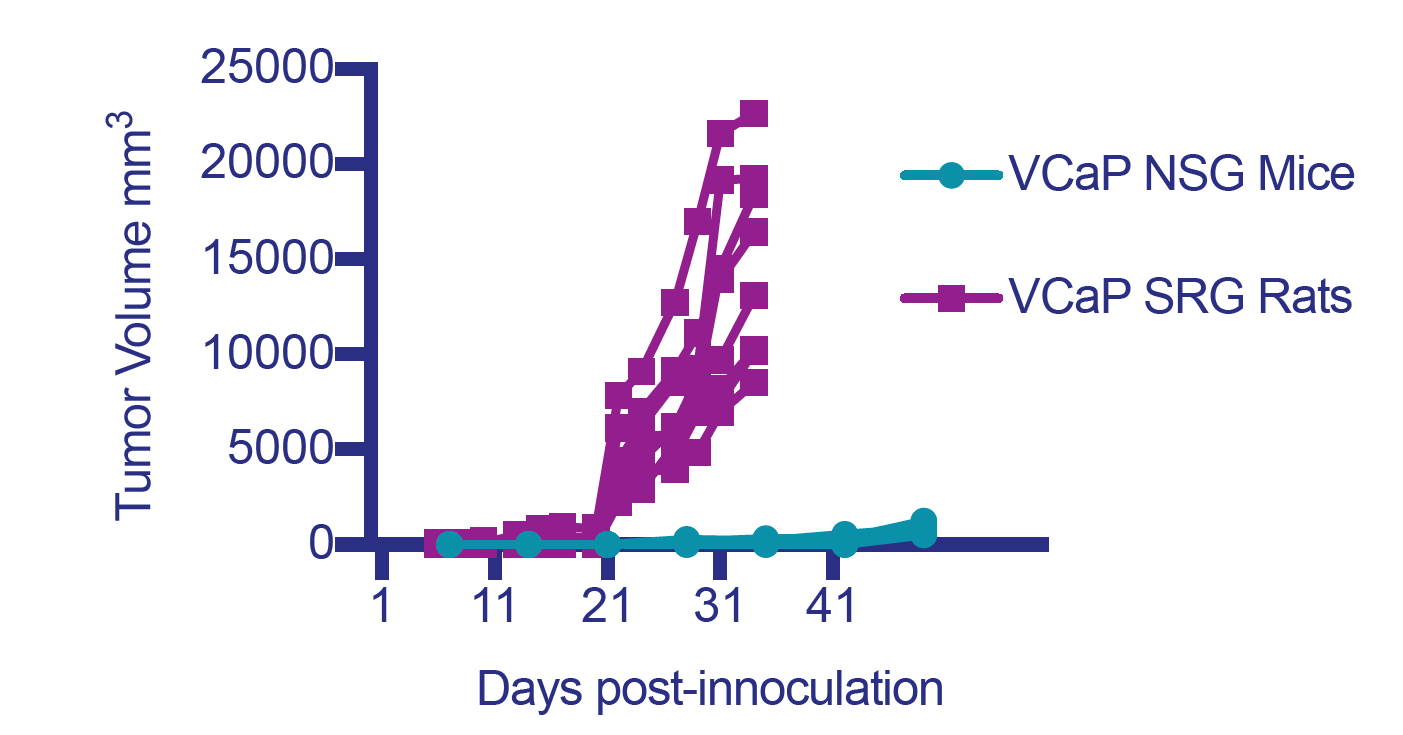
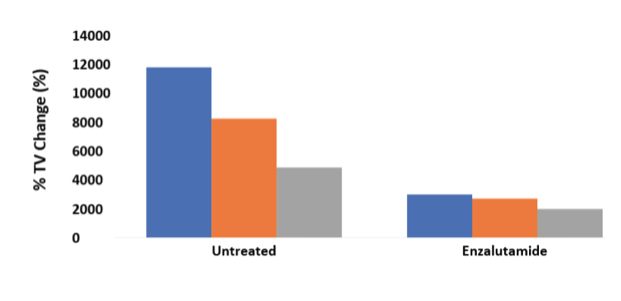
In SCID mice many xenografts show low, non-existent or inconsistent engraftment efficiency. Engraftment efficiencies in 5-20% range are quite common; making it challenging or impossible to generate a cohort of animals with similar tumor sizes for an efficacy study. The OncoRat has demonstrated improved take rates and kinetics enabling effective in vivo studies using tumor models not previously feasible. See our review White Paper on a recent Clinical Cancer Research publication demonstrating engraftment & growth of difficult VCaP & MDVR VCaP prostate cell lines.
Get to efficacy studies & data faster
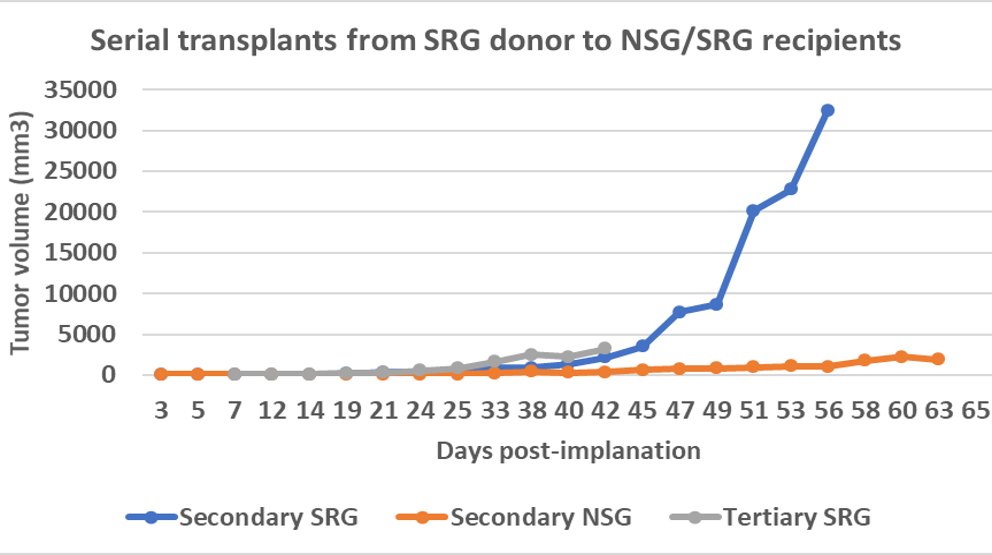
OncoRat grows ~10x larger tumors than SCID mice and produces larger samples for analysis. As such, patient derived xenograft (PDX) material may be expanded faster, getting to efficacy studies in fewer passages and a shorter amount of time in OncoRat.
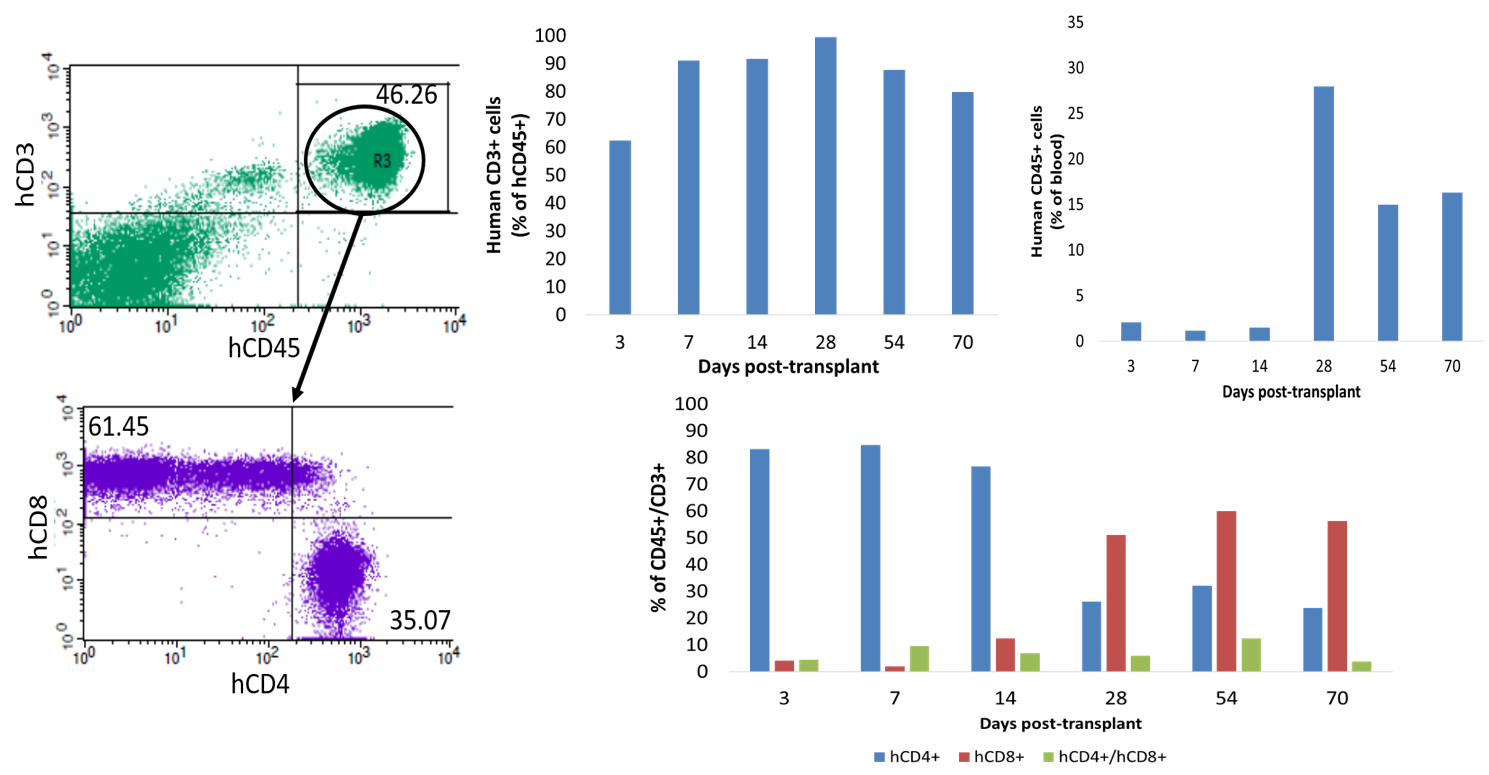
The first humanized immune system rat
SRG rats demonstrate robust immune-system humanization with peripheral blood mononuclear cells. Humanized SRG rats may have advantages in immuno-oncology, especially for tumor models with low take-rates in humanized mice.
Precise data and cost savings with efficacy & safety in one animal
The rat is a larger animal and is the preferred model for translational physiology and toxicology (Iannaccone & Jacob. Rats! Disease Models & Mechanisms. 2009;2(5-6):206-210). The rat’s size is appropriate for multiple dosing, particularly by routes other than oral, and for collection of multiple blood samples, which enable investigators to assess efficacy, systemic toxicity and PK/PD profile in a single animal over longer time periods. Traditionally, efficacy studies would take place in mouse models with the corresponding safety studies being conducted in rat; the resulting data comparisons are less than ideal.