Chemically Induced Diabetes
Chemically induced obesity models provide reliable platform to study the underlying mechanisms of diabetes and related metabolic disorders and are an effective tool for testing novel therapeutics and interventions aimed at treating diabetes.
- Streptozotocin (STZ) induced type 1 diabetes.
- High fat diet (HFD) and STZ-induced type 2 diabetes Range finding or maximum tolerated dose studies

Genetic Animal Models
Our flexible services include standard genetic models of type 2 diabetes, glucose intolerance, and hyperinsulinemia (i.e. ZDF) and custom novel genetically engineered models. Learn more about our transgenic rat and mouse model creation services or read more about our Mc4r KO rat case study below.
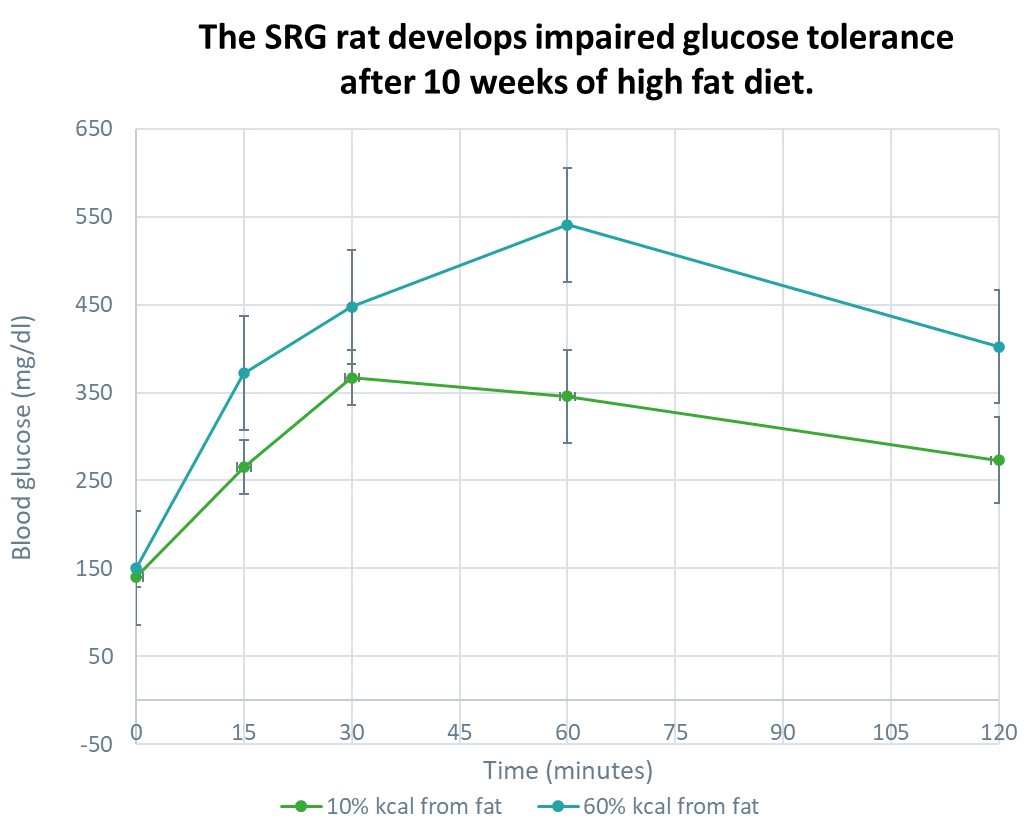
Cell Based Therapies in the SRG Rat
SRG OncoRat, developed using Hera Biolabs’ advanced gene editing technology, is a SCID rat on the Sprague-Dawley background that harbors a double knockout for the Rag2 and Il2rgamma genes. Similar to industry leading NSG mice, OncoRat demonstrates enhanced immunodeficiency, lacking B-cells, T-cells, and NK-cells.
Because the SRG rat is highly immunodeficient it is ideal for testing cell therapies in combination with standard methods of inducing an obesity or diabetes phenotype.
Available Assessments & Assays
Glucose Homeostasis
Insulin tolerance test (ITT; IP or IV)
Glucose tolerance test (GTT; PO, IP, or IV)
Glucose-stimulated insulin secretion (GSIS; PO, IP, or IV)
Food intake, water intake, fecal output, fecal bomb calorimetry
Body composition (carcass):
Whole-body chemical carcass analysis
Dual-energy X-ray absorptiometry (DXA)
Quantitative magnetic resonance (QMR)
Dyslipidemia:
Total cholesterol, HDL, & LDL
Triglycerides
Free fatty acids
ELISA, Luminex®, and Meso Scale Discovery for Hormone Quantification
RNA, Protein, and Lipid Isolation
Mc4r KO FatRat™ Model
A Case Study
Hera (formerly as Transposagen Biopharmaceuticals) created a novel genetically engineered rat model for obesity research, coined The FatRat™, which has a mutation in the melanocortin 4 receptor (Mc4r), is the gene most commonly involved in human obesity. The FatRat™, develops obesity due to hyperphagia (overeating), as well as hyperinsulinemia and elevated leptin levels.

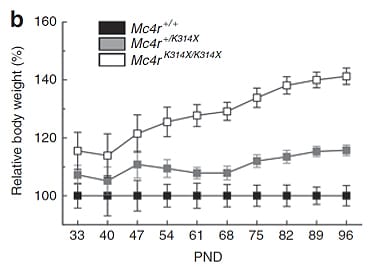
Relative body weight of Mc4r+/+ control wild-type rats (n = 6), Mc4r+/K314X (n = 14) heterozygous rats, and Mc4rK314X/K314X homozygous rats (n = 12) rats during development. Obesity 2012
DISSOCIATION OF OBESITY FROM HYPERTENSION MAKES THE FATRAT™ AN IDEAL COMPLIMENT TO THE ZUCKER DIABETIC FATTY (ZDF) RAT
Obesity is a major cause of hypertension, but links between the obese and hypertensive states remain incompletely understood. Despite being profoundly obese and insulin resistant, FatRats™ are normotensive. In response to ganglionic blockade with mecamylamine, blood pressure and hindlimb resistance fell more in FatRats™, suggesting that sympathoactivation of the vascular was still evident, despite the absence of hypertension (Phys Rep 2013).
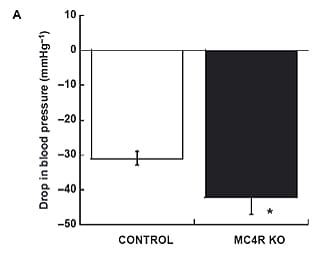
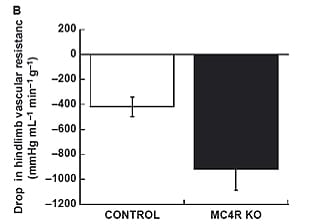
In vivo hemodynamic responses to ganglionic blockade with mecamylamine (2 m/kg). (A) is drop in blood pressure in mmHg. (B) is the drop in hindlimb vascular resistance
- Mul et al. Melanocortin Receptor 4 Deficiency Affects Body Weight Regulation, Grooming Behavior, and Substrate Preference in the Rat. Obesity. 2012. (Full text)
- Roth et al. Interactions of amylinergic and melanocortinergic systems in the control of food intake and body weight in rodents. Diabetes Obesity and Metabolism. 2012. (Full text)
- Stepp et al. Vascular effects of deletion of melanocortin-4 receptors in rats. Phys Rep 2013. (Full text)
Off-the-self animals are no longer available.
Choosing the Right Preclinical Rodent Model for Obesity Research
Diabetes is a complex metabolic syndrome that affects millions of people worldwide. In preclinical research, selecting the appropriate animal model is crucial to studying the pathophysiology of diabetes and developing effective therapeutic interventions. Three commonly employed models include STZ-induced diabetes, diet-induced models, and genetically modified strains. Each model offers unique advantages and limitations, and understanding when to use them is essential for obtaining meaningful results.
STZ-Induced Diabetes Model: The streptozotocin (STZ)-induced diabetes model involves the administration of STZ, a naturally occurring compound that selectively destroys pancreatic beta cells, leading to insulin deficiency. This model mimics type 1 diabetes characterized by absolute insulin deficiency. STZ-induced diabetes is often used when studying autoimmune mechanisms, pancreatic beta cell transplantation, or evaluating novel therapeutic approaches aiming to restore insulin production. Researchers can control the severity of diabetes by adjusting the STZ dose, allowing for different degrees of beta cell destruction.
Diet-Induced Models: Diet-induced models involve feeding animals a high-fat or high-sugar diet, leading to obesity, insulin resistance, and eventual beta cell dysfunction. These models closely resemble type 2 diabetes, which is characterized by insulin resistance and relative insulin deficiency. Diet-induced models are versatile and widely used, as they reflect the lifestyle and dietary factors contributing to the development of type 2 diabetes in humans. They are particularly useful for studying metabolic abnormalities, glucose homeostasis, and exploring the effects of dietary interventions or pharmacological treatments.
Genetically Modified Strains: Genetically modified strains involve altering the genetic makeup of animals to mimic specific aspects of diabetes, such as impaired insulin signaling, defective beta cell function, or disrupted glucose metabolism. These models enable researchers to investigate the precise molecular mechanisms underlying diabetes. Genetically modified strains are valuable for understanding the genetic basis of diabetes and evaluating potential therapeutic targets. However, it is important to note that these models may not fully capture the complexity of the human disease and should be complemented with other models for comprehensive insights.
The choice of model depends on the specific research question and the aspect of diabetes being investigated and considering the research objectives, disease phenotype, and translational relevance, researchers can select the most appropriate model or you can let our scientific team provide expertise on the best model for your research goals.
Additional considerations:

