Take the first step in determining the safest and most appropriate concentrations for your drug candidates.
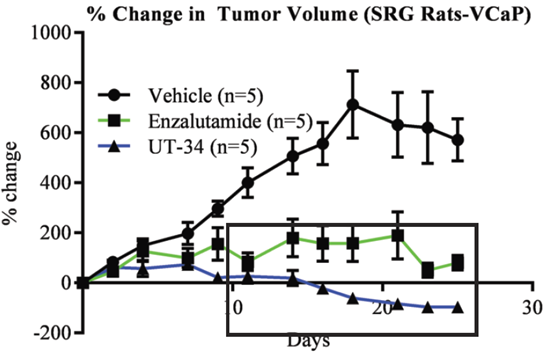
We provide drug efficacy studies in a variety of in vivo models, including multiple immunodeficient mouse strains and our SRG OncoRat®, a rat model with unsurpassed immunodeficiency and ease-of-use.
Our experience extends to humanized immune system (PBMC or CD34+) models as well as novel transgenic models that we engineer and validate for our clients.
Learn more about what makes us different.
Preclinical Drug Development Studies
Preclinical drug discovery and development aims to determine if a drug is ready for clinical trials through preclinical in vitro and in vivo safety assessments.
Target identification and validation often involve high-throughput screening as well as high content screening to determine if a target can be manipulated by the drug.
Pharmacokinetics or Pharmacodynamics
Our flexible services include serial blood and tissue/organ collection in both mice and rat models – combined with multiple routes of dosing, including oral gavage, IV, IP, and continuous infusion(s).
Our SRG rat enables serial tumor biopsies to study intra-tumor drug exposure (PD). Check out our white paper for more information.
Preclinical Oncology Services
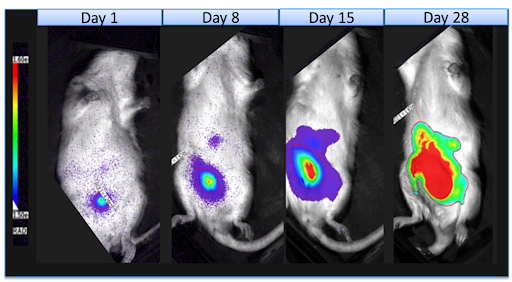
Our passion is preclinical oncology and immuno-oncology studies, including syngeneic models and cell-derived and patient-derived cancer xenografts.
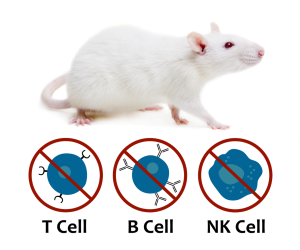
The SRG OncoRat
The SRG OncoRat is highly immunodeficient, completely lacking circulating B-cells, T-cells, and NK-cells. It boasts high xenograft and PDX tumor engraftment rates and more uniform growth kinetics than seen in mice.
Its Sprague-Dawley background enables combined efficacy and in vivo lead optimization parameters to identify meaningful risks early and accelerate your preclinical program.
Xenograft vs. PDX
Researchers are often asking our scientific team about how to choose between using a cell line xenograft and a patient derived xenograft (PDX). This article aims to summarize the pros and cons of each approach and allow you to identify which model type is the best fit for your drug development needs.
Most preclinical oncology drug efficacy studies are conducted in cell line xenografts because they are more consistent, characterized, and cost effective. However, some researchers believe that using a patient derived xenograft model could provide more translation data by preserving the original tumor complexity and heterogeneity.
Read more about which model might be right for your evaluating your therapeutic candidates.
Choosing the Right Preclinical CRO
Finding the right partner for outsourcing your pharmacology, toxicology or drug efficacy studies is extremely important. As a non-GLP preclinical CRO, our specific strengths lie in early-stage drug development for small molecules, biologics, cell therapies, or gene therapies.
Hera currently has the capacity to immediately start your studies with no wait-times for our contract research services. The biopharmaceutical companies and academic institutes that we work with rely on our rapid execution of well-designed studies that produce high-quality data.
In order to advance your new drug through pre-IND GLP toxicology studies into the IND or regulatory process and finally clinical trials, it is essential to start on the right foot with a preclinical CRO that delivers high-quality data and flexible services. Our comprehensive toxicology contract laboratory services are focused on the non-GLP in vivo studies that are essential for drug screening in mice and rats. Our approach includes rigorous compliance with regulatory standards and Hera is actively pursuing further accreditations in order to provide highly accurate and reliable test results which allow clients to make informed decisions regarding the safety and efficacy of their compounds.
Our immunodeficient SRG rat has the versatility to allow for the combination of efficacy studies that require a xenograft/allograft of a tumor, tissue, or medical device as well as PK/PD or DMPK toxicity endpoints all in the preferred Sprague Dawley background.
Can’t find the right preclinical model? Let Hera engineer the perfect cell-line or transgenic animal model for evaluating your development candidate or therapeutic. From in vivo gene delivery to new surgical techniques- there is no problem that cannot be solved by our team of scientists.